Solving a Magnesium Mystery in Rechargeable Battery Performance
Study reveals surprising, bad chemical reactivity in battery components previously considered compatible.
The Science
Packing more energy into smaller rechargeable batteries could extend the range of electric vehicles. Magnesium-based batteries have potential but also have chemical roadblocks. Now, a team of Molecular Foundry scientists and users has discovered a new kind of chemical reactivity. The self-stabilizing, thin oxide surface layer that forms on the magnesium electrode has defects. These defects can expose underlying magnesium ions. The ions trap molecules from the battery’s liquid electrolyte. The battery begins to fail before it’s even charged. Previously, scientists thought the next-generation electrode and electrolyte were compatible.
The Impact
The discovered reactions degrade battery performance even before the battery can be charged. The findings could be relevant to other battery materials and guide the design of next-gen batteries away from such pitfalls.
Summary
Rechargeable batteries based on magnesium, rather than lithium, have the potential to extend electric vehicle range by packing more energy into smaller batteries. But unforeseen chemical roadblocks have slowed scientific progress. The places where solid meets liquid—where the oppositely charged battery electrodes interact with the surrounding chemical mixture known as the electrolyte—are the known problem spots.
Now, a research team has discovered a surprising set of chemical reactions involving magnesium that degrade battery performance even before the battery can be charged up. The findings could be relevant to other battery materials and could steer the design of next-generation batteries toward workarounds that avoid these newly identified pitfalls.
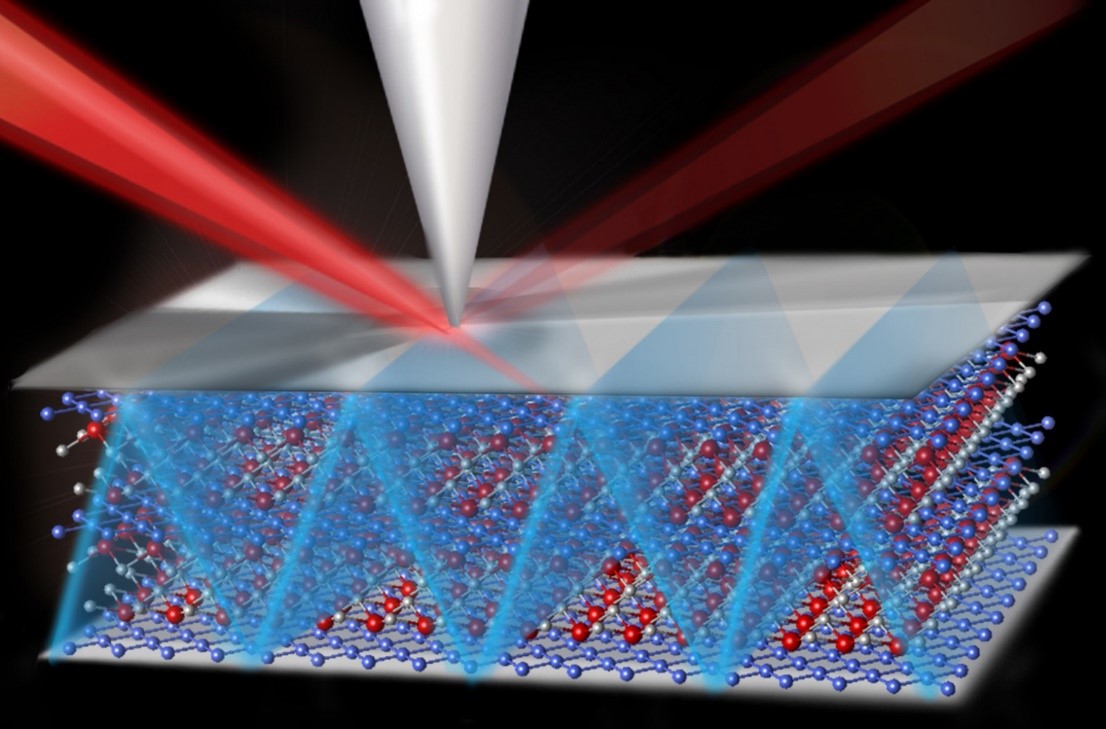
The team used X-ray experiments, theoretical modeling, and supercomputer simulations to develop a full understanding of the chemical breakdown of a liquid electrolyte occurring within tens of nanometers of an electrode surface that degrades battery performance.
The battery they were testing featured magnesium metal as its negative electrode (the anode) in contact with an electrolyte composed of a liquid (a type of solvent known as diglyme) and a dissolved salt, Mg(TFSI)2. Molecular Foundry researchers developed detailed simulations of the point where the electrode and electrolyte meet, known as the interface, indicating that no spontaneous chemical reactions should occur under ideal conditions, either. The simulations, though, did not account for all of the chemical details.
The team employed a unique X-ray technique developed recently at the Advanced Light Source, called APXPS (ambient pressure X-ray photoelectron spectroscopy). This new technique is sensitive to the chemistry occurring at the interface of a solid and liquid, which makes it an ideal tool to explore battery chemistry at the surface of the electrode, where it meets the liquid electrolyte.
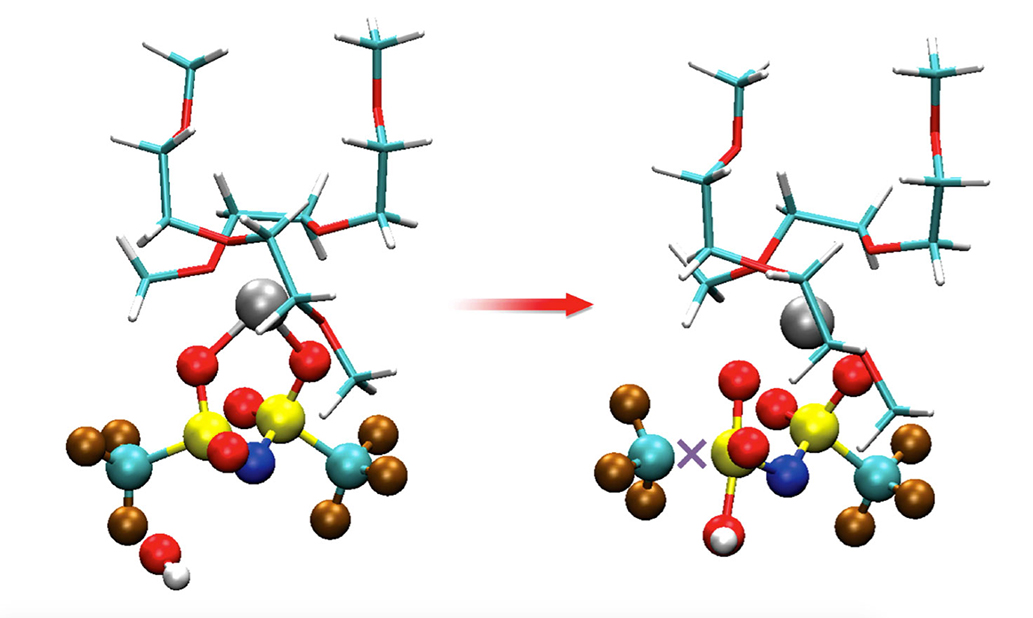
What they determined is that the self-stabilizing, thin oxide surface layer that forms on the magnesium has defects and impurities that drive unwanted reactions. A further round of simulations, which proposed possible defects in the oxidized magnesium surface, showed that defects in the oxidized surface layer of the anode could expose magnesium ions that then act as traps for the electrolyte’s molecules. The results could be relevant to other types of battery materials, too, including prototypes based on lithium or aluminum metal.
Contact
David Prendergast
Molecular Foundry, Berkeley Lab
dgprendergast@lbl.gov; (510) 486-4948
Funding
This work was supported by Joint Center for Energy Storage Research, an Energy Innovation Hub, and the Nanostructures for Electrical Energy Storage (NEES), an Energy Frontier Research Center, both funded by the Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences. Portions of this work were supported by a user project at the Molecular Foundry, an Office of Science scientific user facility, and its compute cluster (Vulcan) managed by the High Performance Computing Services Group at Lawrence Berkeley National Laboratory (LBNL), and portions of this work used the computing resources of the National Energy Research Scientific Computing Center, a scientific user facility at LBNL, all of which are supported by the DOE Office of Science. The researchers also used resources at the Advanced Light Source, a scientific user facility which is supported by the Director, DOE, Office of Science, Office of Basic Energy Sciences. Q.L. would like to acknowledge the support from the National Natural Science Foundation of China.
Publications
Y. Yu, A. Baskin, C. Valero-Vidal, N.T. Hahn, Q. Liu, K.R. Zavadil, B.W. Eichhorn, D. Prendergast, and E.J. Crumlin, “Instability at the electrode/electrolyte interface induced by hard cation chelation and nucleophilic attack.” Chemistry of Materials 29, 8504(2017). [DOI: 10.1021/acs.chemmater.7b03404]
Related Links
Berkeley Lab press release: Scientists solve a magnesium mystery in rechargeable battery performance
Highlight Categories
Program: ASCR , BES , SUF , EFRCs , Hubs
Performer: University , DOE Laboratory , SC User Facilities , ASCR User Facilities , NERSC , BES User Facilities , ALS , Foundry
Additional: Collaborations , International Collaboration