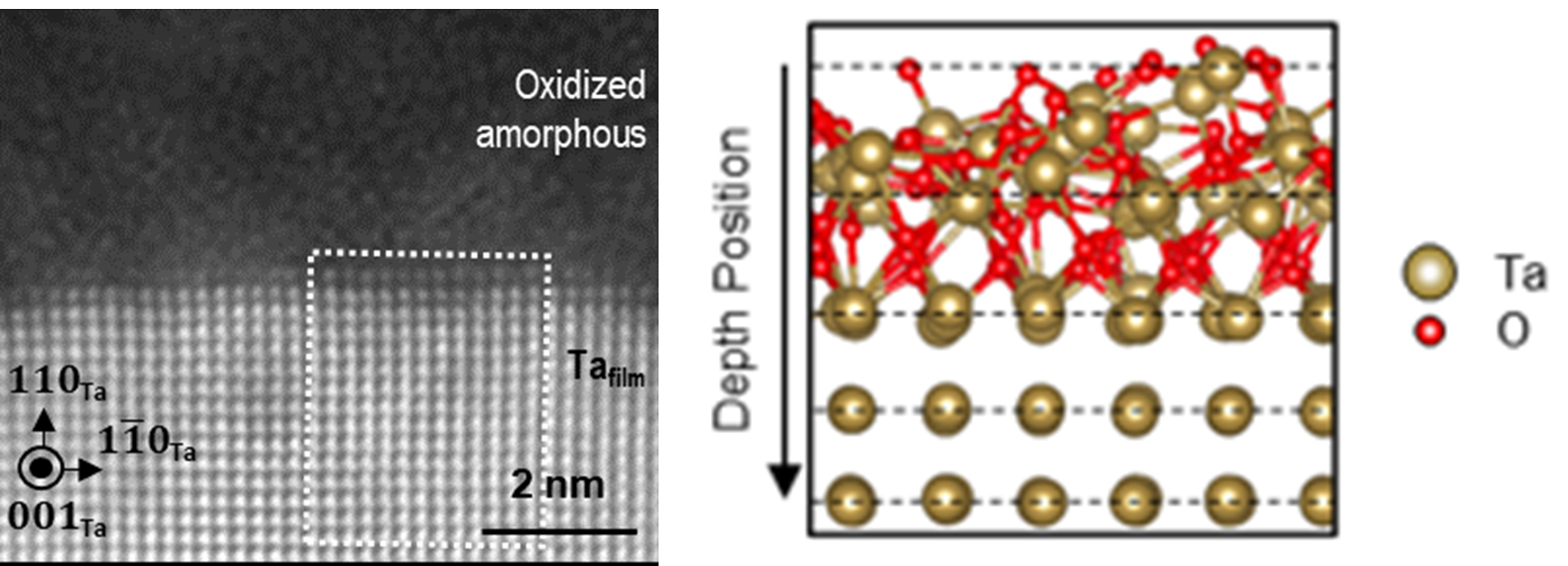
Researchers Build an Atomic-Level Model of Oxidization on the Surface of Tantalum Film
Advanced electron microscopy and first principles calculations reveal atomic motifs at the oxidized surface of superconducting tantalum film.

Advanced electron microscopy and first principles calculations reveal atomic motifs at the oxidized surface of superconducting tantalum film.
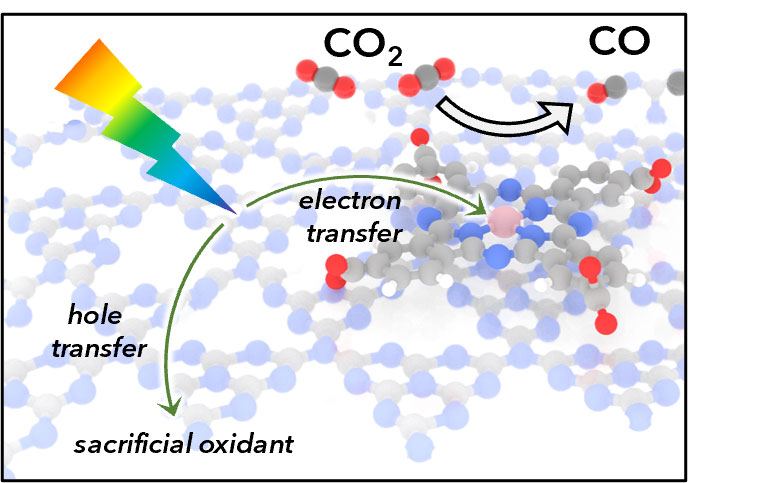
A molecular catalyst integrated with a carbon nitride semiconductor harvests sunlight to rapidly and selectively convert carbon dioxide into carbon monoxide.
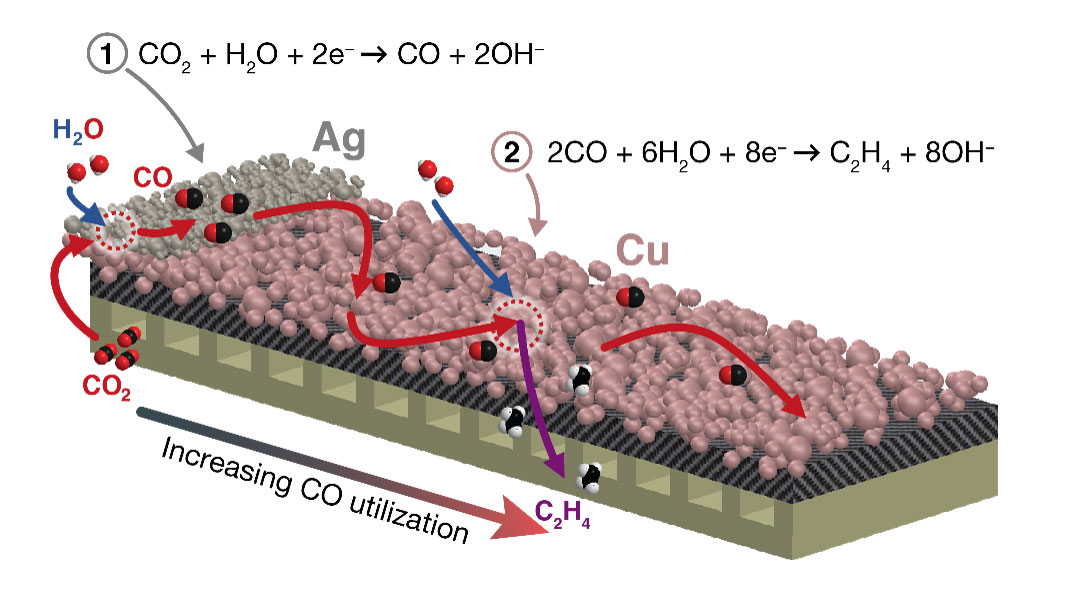
Electrode engineering produces unprecedented selectivity, and high rates of carbon dioxide reduction to multicarbon products.
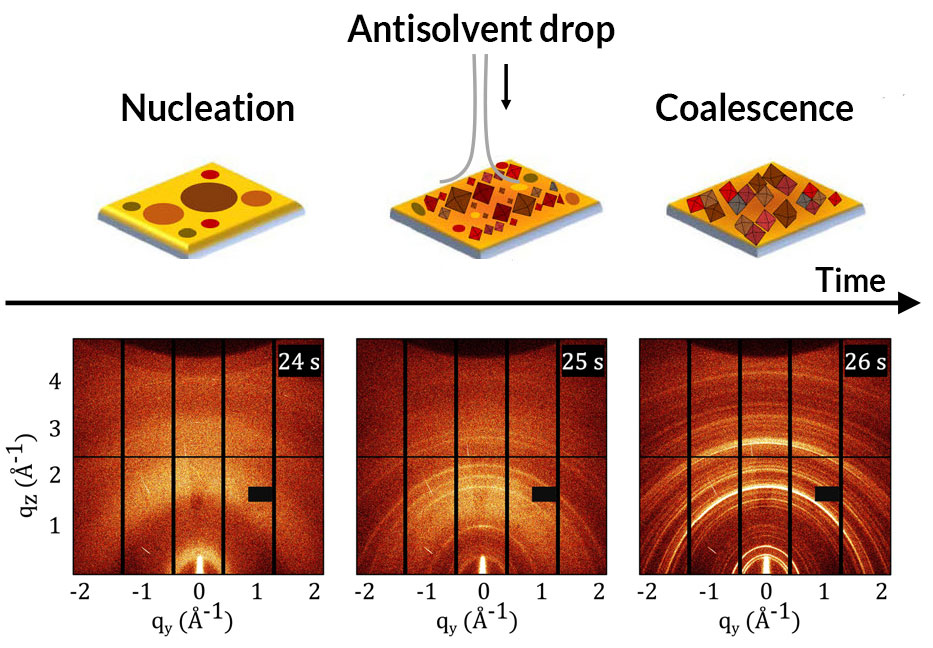
Using two methods is better than one when it comes to observing how solar cells form and improving cell properties.
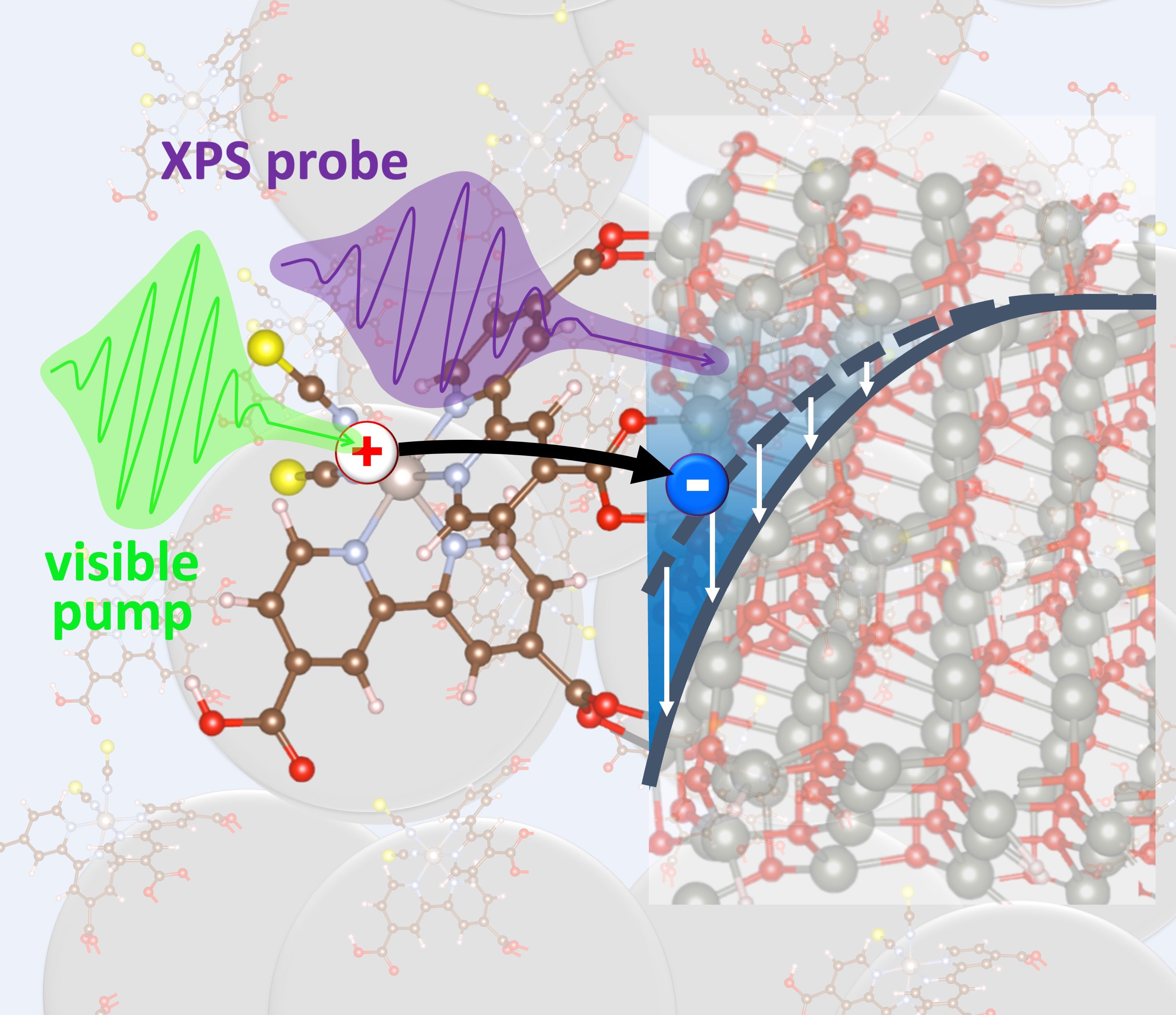
Monitoring photo-excited electrons in real time with nanometer sensitivity reveals strengths and weaknesses in a common light-harvesting material.
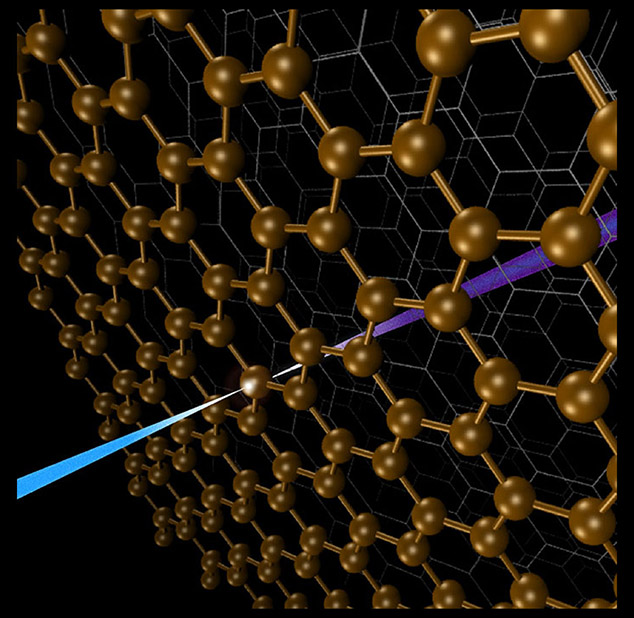
Element-selective method reveals interfacial properties of materials used for water purification, catalysis, energy conversion, and more.
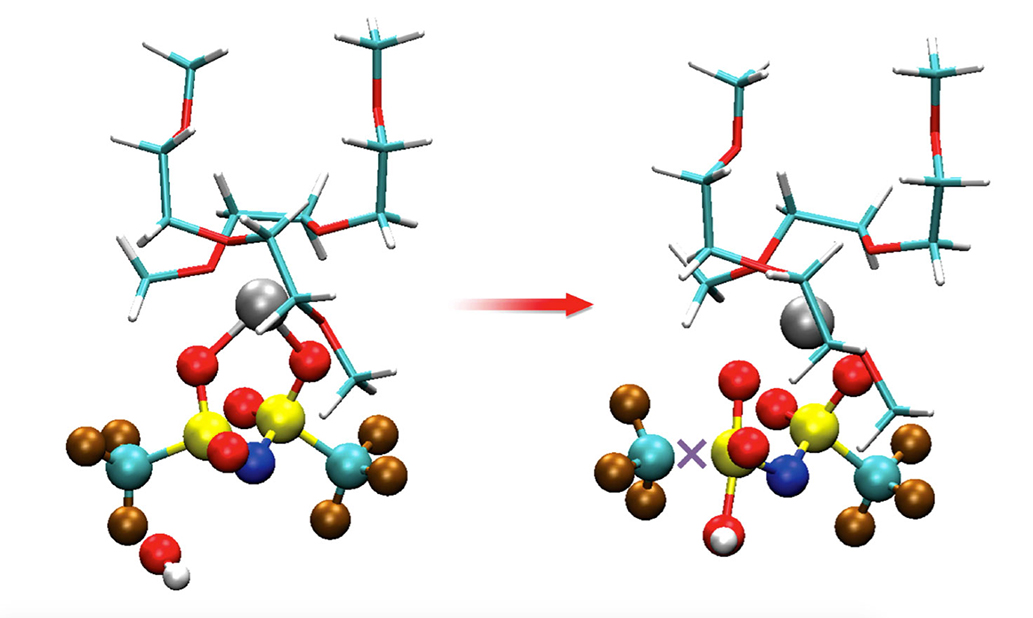
Study reveals surprising, bad chemical reactivity in battery components previously considered compatible.
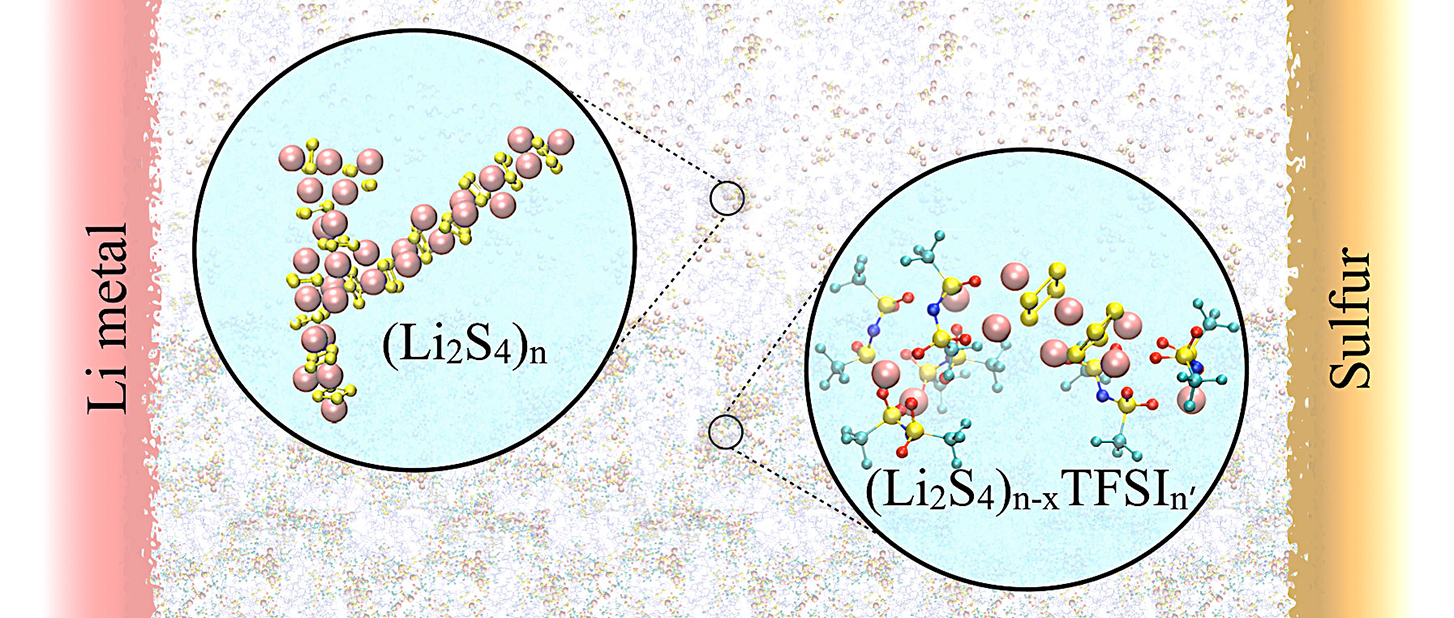
Optimizing lithium-sulfur battery electrolytes for long life.
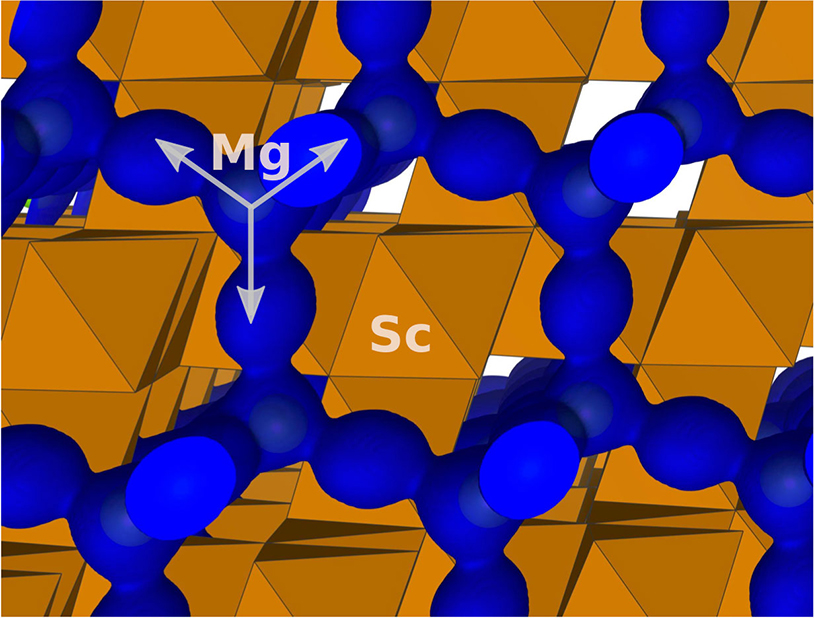
Magnesium ions move very fast to enable a new class of battery materials.
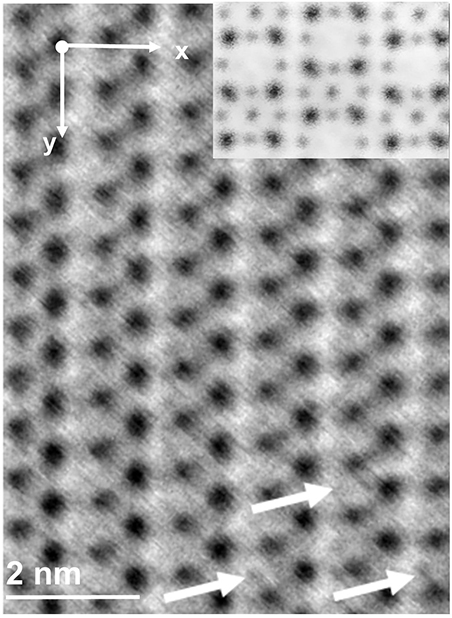
Scientists directly see how the atoms in a magnesium-based battery fit into the structure of electrodes.

New materials could turn water into the fuel of the future.
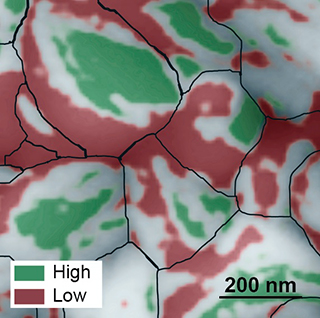
Nanoscale images by Molecular Foundry researchers yield surprise that could push solar cell efficiency to 31 percent.