Graphene Ribbons Result in 100-Fold Increase in Gold Catalyst’s Performance
Bottom-up synthesis of tunable carbon nanoribbons provides a new route to enhance industrial, automotive reactions.
The Science
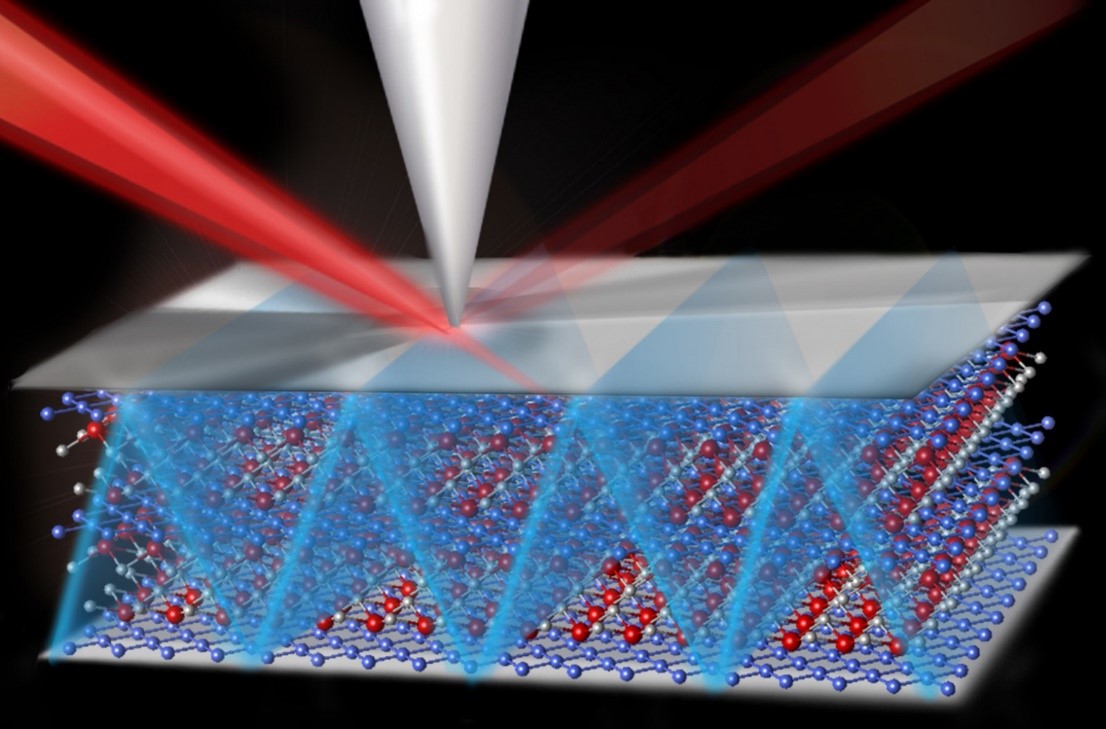
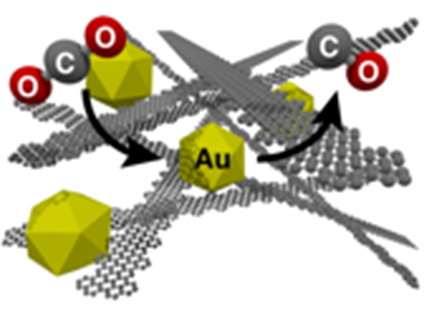
Catalysts drive a wide range of commercial processes—and are critical in energy systems. The efficiency of the catalysts, typically small atomic clusters with high surface areas, depends on many factors. These factors include the materials that hold the catalysts in place during use. In this research, scientists used precisely synthesized one-atom-thick carbon (graphene) nanoribbons as the support for a gold catalyst. Changing the support changed the chemical environment. The result was a faster and more efficient catalyst. Specifically, the catalyst was over 100-fold faster than the same catalyst on the traditional carbon support.
The Impact
One of the uses of catalysts under current development is making liquid fuels. To make fuels, carbon dioxide is converted to carbon monoxide that could be combined with hydrogen. In this research, the graphene ribbons represent a more efficient, stable option to turn carbon dioxide to carbon monoxide.
Summary
Catalysts—specialized chemicals that make catalytic converters function and control a host of chemical processes in industry—are used in a complex chemical environment that affects their stability, selectivity, and activity. Controlling the environment to optimize catalyst performance represents a challenge to catalyst system designers. The researchers demonstrated that the performance of gold nanoparticle catalysts could be dramatically enhanced when a one-atom-thick ribbon of carbon, a graphene nanoribbon, supports the catalysts. These composite catalysts can be used to transform carbon dioxide to carbon monoxide, a first step in the conversion of carbon dioxide into fuels or other useful chemicals. The experiments reveal that the structural and electronic properties of the catalyst composite increase the active surface area of the catalyst and substantially lower the voltage needed to reduce the carbon dioxide to carbon monoxide. The new materials also markedly improve the stability of the catalyst and greatly increase the total catalytic output (greater than a 100-fold improvement over traditional amorphous carbon-gold nanoparticle composites). The fact that the structural and electronic properties can be tuned through rational chemical synthesis methods gives researchers a way to control the catalytic environment. The experiment conclusively demonstrates that nanoparticle catalysts can be controlled and greatly improved by changing the chemical surroundings created by the support material.
Contact
Prof. Felix Fischer
University of California, Berkeley
ffischer@berkeley.edu
Funding
U.S. Department of Energy, Office of Science, Basic Energy Sciences including use of the Molecular Foundry, an Office of Science user facility
Publications
C. Rogers, W.S. Perkins, G. Veber, T.E. Williams, R.R. Cloke, and F.R. Fischer, “Synergistic enhancement of electrocatalytic CO2 reduction with gold nanoparticles embedded in functional graphene nanoribbon composite electrodes.” Journal of the American Chemical Society 139, 4052-4061 (2017). [DOI: 10.1021/jacs.6b12217]
Highlight Categories
Performer: University , SC User Facilities , BES User Facilities , Foundry