Bringing Order to Defects - Making Way for Oxygen to Move
New metal oxide material works at temperatures low enough to improve fuel cell efficiency.
The Science
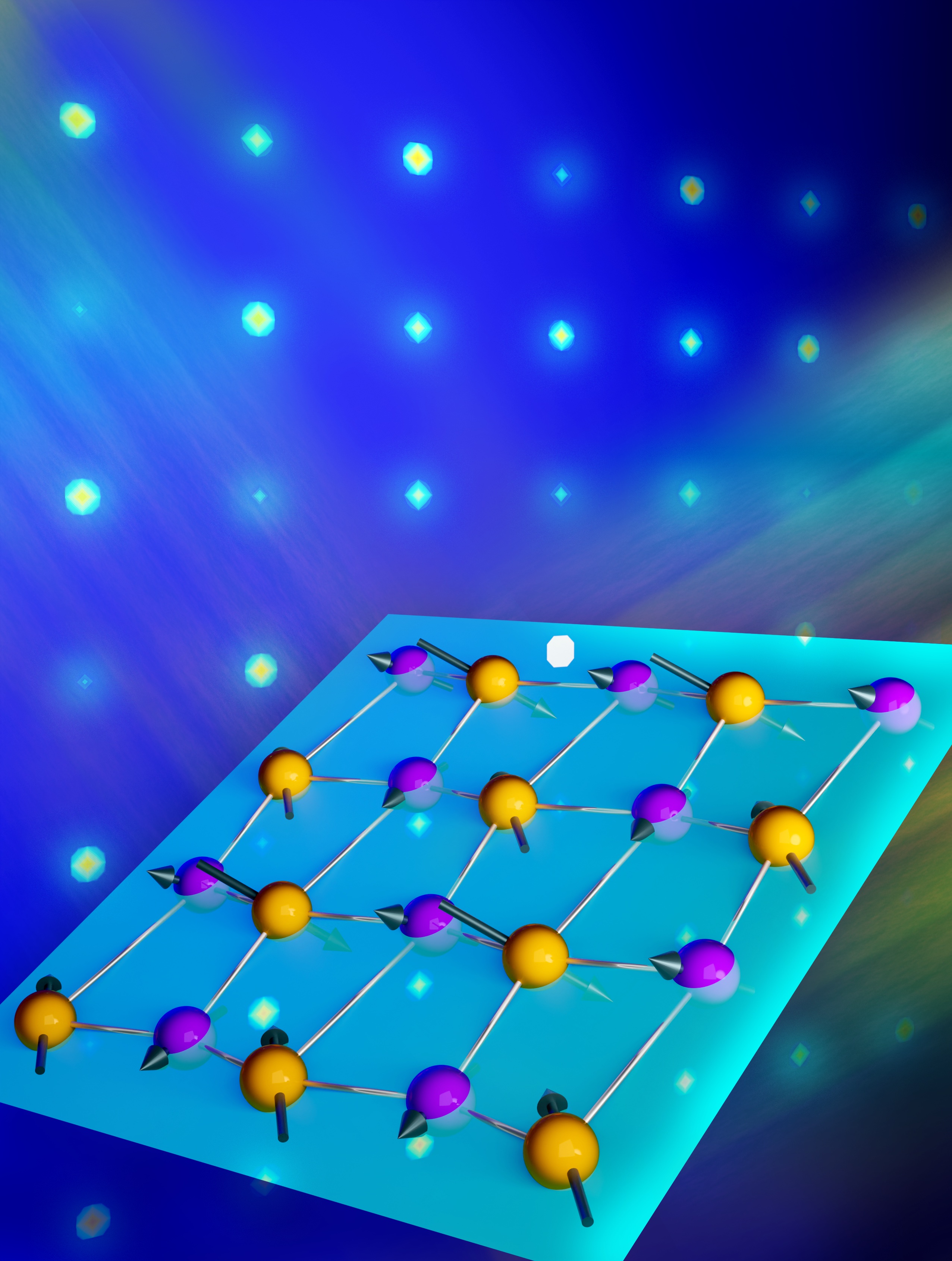
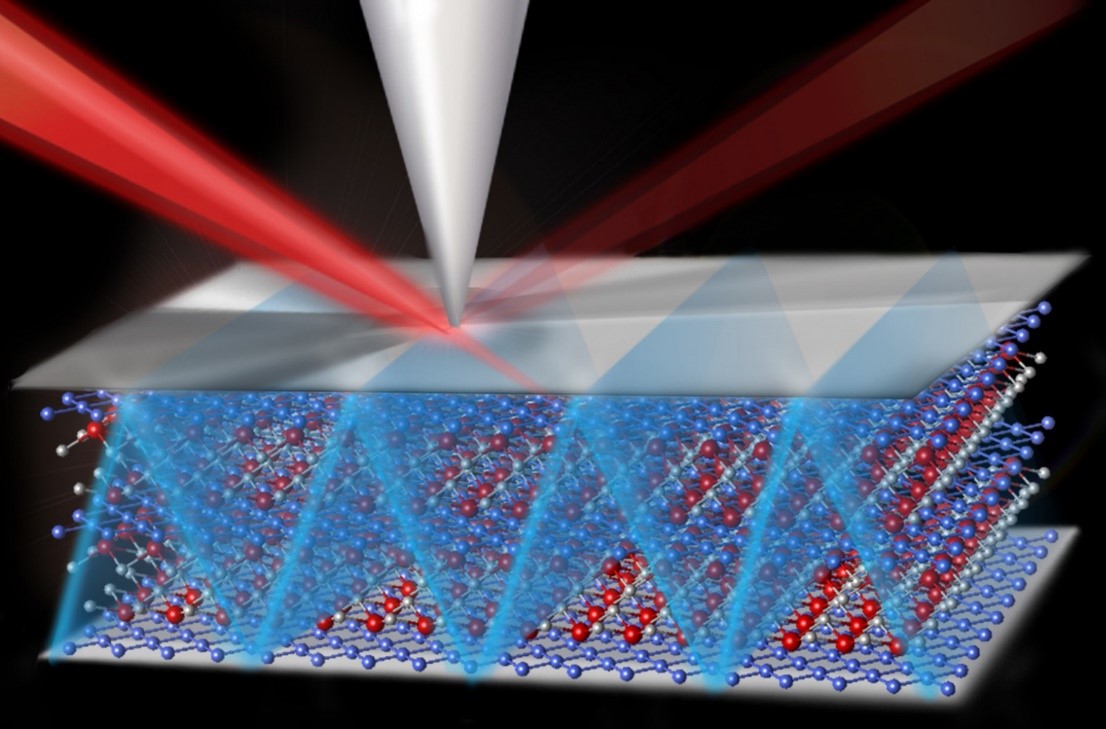
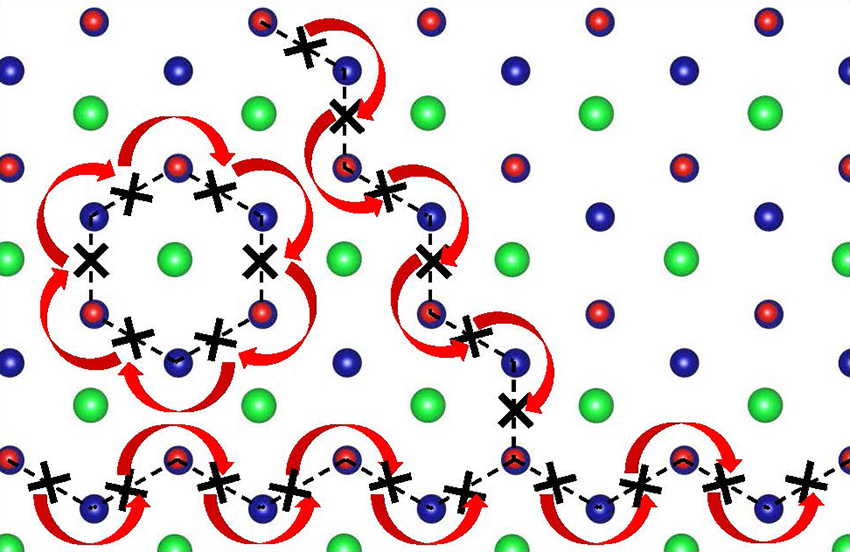
A new metal oxide was discovered whose atomic structure includes highly ordered arrays of missing oxygen atoms (also referred to as oxygen-deficiencies or vacancies). This structure allows oxygen ions (O2–) to move through the material quickly and easily at low temperatures (~250°C).
The Impact
Materials that allow easy movement of oxygen ions are essential for solid oxide fuel cells. The material discovered in this research could enable more efficient solid oxide fuel cells to operate at much lower temperatures than current technology (~800°C).
Summary
Metal oxides are important materials found in many energy technologies such as fuel cells, superconductors, and thermoelectric systems. Most metal oxides contain oxygen deficiencies or vacancies as point defects which are often uniformly distributed in the material. A challenging goal is controlled generation and positioning of these oxygen deficiencies to create novel structures and functional properties. Researchers at the Pacific Northwest National Laboratory have accomplished just that in their discovery of a new, non-stoichiometric metal oxide SrCrO2.8. While attempting to prepare thin films of stoichiometric SrCrO3.0, they found that a non-stoichiometric form with the composition SrCrO2.8 is formed instead. This new material contains ordered arrays of SrO2 planes interleaved between layers of tetrahedrally coordinated Cr+4 ions and separated by ~1nm. Moreover, when mildly heated in air, it reversibly transforms from a semiconducting form with rhombohedral (diamond-like) structure to a metallic form with a so-called cubic perovskite structure. The ordered oxygen vacancies in the rhombohedral form allow oxygen ions to diffuse through the material quickly and easily at low temperatures (~250°C). This property is exceedingly important for solid oxide fuel cell technology which currently requires very high operating temperatures (~800°C). First-principle calculations provided insights into the formation and stability of the non-stoichiometric SrCrO2.8 and how oxygen ions could move so easily through the material. The aggregation of oxygen vacancy defects into ordered arrays is a property of interest for not only more efficient solid oxide fuel cells but also would be useful for other applications such as thermoelectrics.
Contact
Scott Chambers
Pacific Northwest National Laboratory
SA.Chambers@pnnl.gov
Funding
DOE Office of Science, Basic Energy Sciences program. The research included staff and use of the facilities supported by the Environmental Molecular Sciences Laboratory, a DOE Office of Science user facility supported by the Biologic and Environmental Sciences program. Computational facilities were supported by Pacific Northwest National Laboratory.
Publications
K. H. L. Zhang, P. V. Sushko, R. Colby, Y. Du, M. E. Bowden and S. A. Chambers, “Reversible Nano-structuring of SrCrO3-d through Oxidation and Reduction at Low Temperature”, Nature Communications, August 18 (2014). [DOI: 10.1038/ncomms5669]
Related Links
http://www.sciencedaily.com/releases/2014/09/140910185908.htm
www.innovations-report.com/html/report/materials-science/angling-chromium-to-let-oxygen-through.html
http://www.rdmag.com/news/2014/09/angling-chromium-let-oxygen-through
http://semiconductors.einnews.com/pr_news/223084921/angling-chromium-to-let-oxygen-through
Highlight Categories
Performer: DOE Laboratory , SC User Facilities , BER User Facilities , EMSL