Capturing the Chemistry of Radium-223 for Cancer Treatment
Understanding radium’s chemistry increases the likelihood of using it for targeted alpha therapy in soft tissue.
The Science
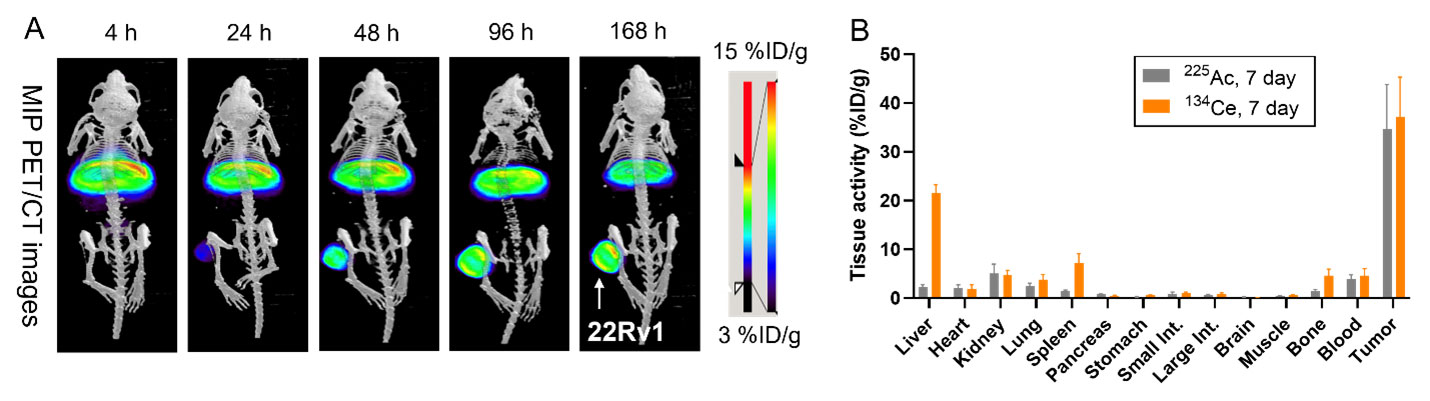
Scientists need a better understanding of the chemistry of radium to be able to target the isotope radium-223 (Ra-223) to cancer cells. Once delivered, Ra-223 can destroy those cells with alpha particles, a type of radiation. Researchers investigated radium’s chemistry by looking at how it interacts with two chelators, or binding molecules, called macropa and DOTA. Doctors use both chelators in targeted alpha cancer therapy. Through experiments and computer-driven models, the researchers discovered that macropa is the strongest chelator for binding radium identified so far. They also gained information about how the structure and properties of these chelators affect how well they bind to radium.
The Impact
Ra-223 has already been approved by the Food and Drug Administration for treating prostate cancer patients whose cancer has spread to the bone. This research is significant as it means Ra-223 could also treat cancers outside the bone. It gives information about the specific kind of chelators needed to successfully bind this radioactive ion to a molecule that can deliver it directly to cancer in the body. This new knowledge about radium’s properties will help researchers find the best possible chelator to bind Ra-223 and expand its use in cancer therapy. Ra-223 is available from the DOE Isotope Program’s National Isotope Development Center.
Summary
Until now, there have been few efforts to get information on how radium binds with known chelators. To address this gap, scientists at Oak Ridge National Laboratory (ORNL) used experiments and computer-driven models to learn about two state-of-the-art binding molecules, macropa and DOTA. Researchers already knew these two molecules could bind the radium ion in water, but they didn’t know how stable those bonds were. Past studies looked at only simple organic molecules that are less relevant for use in medicine.
The work determined that the Ra−macropa complex had a more stable bond than that of any other studied Ra complexes under normal biological conditions. The Ra−DOTA complex was only moderately stable when the sodium ion was present, but its stability increased under sodium-free conditions. The study showed that, as scientists already had thought, chelators form bonds with radium that are primarily ionic. That means highly charged chelators can form highly stable complexes. But it also highlighted how important it is to look at the effects of sodium and other competing ions present in the body when finding the right chelators for Ra-223-based targeted alpha therapy. Scientists must consider ionic interactions, metal-ion selectivity and the chemical makeup of the chelator when designing molecules to stabilize radium.
Contact
Nikki Thiele
Oak Ridge National Laboratory
Thielena@ornl.gov
Alexander Ivanov
Oak Ridge National Laboratory
ivanova@ornl.gov
Paul Benny
Oak Ridge National Laboratory
bennypd@ornl.gov
Funding
This research was supported by the Department of Energy Isotope Program, managed by the Office of Science for Isotope R&D and Production, and by the ORNL Laboratory Directed Research and Development Program.
Publications
Ivanov, A. S., et al. Elucidating the coordination chemistry of the radium ion for targeted alpha therapy. Chemical Communications 58, 9938 (2022). [DOI: 10.1039/d2cc03156f]
Related Links
Radium chelator researchers working to improve targeted cancer therapies, Chemistry World
ORNL scientist working on a more targeted treatment for cancer, WVLT-TV Knoxville
Highlight Categories
Performer: DOE Laboratory