Revealing the Reactions Behind How Complex Carbon Molecules Form in Space
Understanding how polycyclic aromatic hydrocarbons form can help scientists better understand the origin and evolution of carbon in our galaxy
The Science
Polycyclic aromatic hydrocarbons (PAHs) are a type of compound made of carbon and hydrogen. In chemistry, they may be the critical link between two important species in chemical reactions. One of these types of particles are extremely small carbon-based particles. These carbon-based particles form both when carbon burns without enough oxygen on Earth and near carbon-rich giant red stars. Although PAHs resemble the building blocks of these complex, very small carbon-based particles, scientists don’t yet know how PAHs form at extremely hot temperatures. This study revealed two key high temperature chemical pathways that can lead to the formation of PAHs.
The Impact
Polycyclic aromatic hydrocarbons (PAHs) are associated with the formation of new stars and planets. This study suggests two critical reactions that could lead to PAH growth. Theoretical simulations in past studies had suggested these two mechanisms. However, researchers in this study confirmed them with experiments. They revealed how carbonaceous nanoparticles (extremely small carbon-based particles) can form in combustion, materials synthesis, and deep space. Understanding the fundamental reactions that lead to PAHs will help scientists better understand the origin and evolution of molecules in the universe. In particular, it will provide insight into how carbon-containing molecular compounds formed in our galaxy
Summary
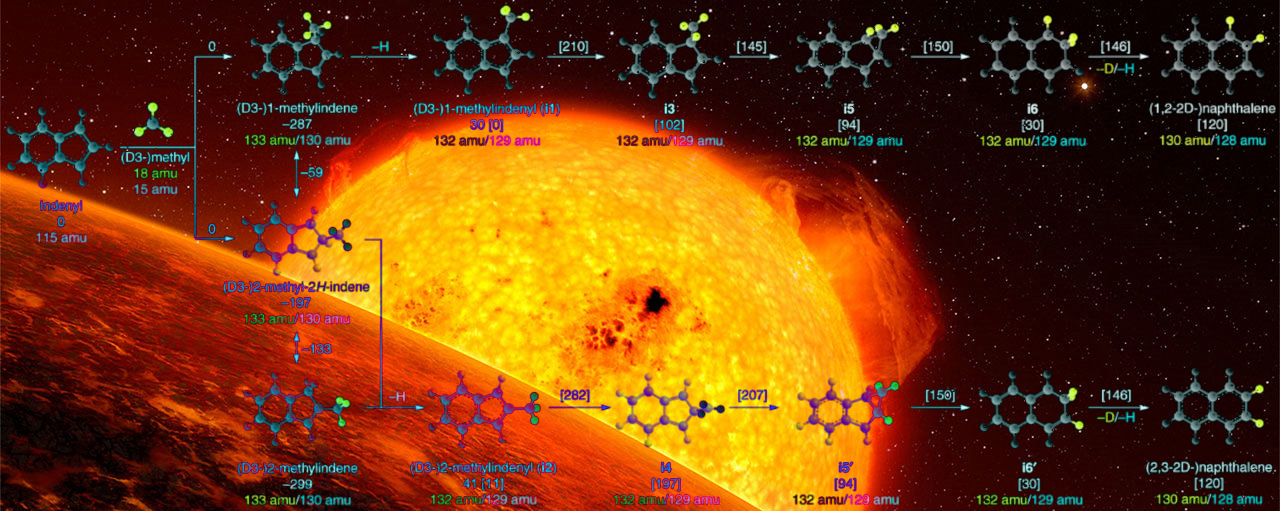
By exploring these reaction mechanisms first of the phenyl radical with biphenyl/naphthalene theoretically and experimentally, we provide compelling evidence on a novel Phenyl‐Addition/dehydroCyclization (PAC) pathway leading to prototype PAHs: triphenylene and fluoranthene. Secondly, radical-radical reactions (RRR) between the methyl and the indenyl species were shown to form the simplest representative of a polycyclic aromatic hydrocarbon: naphthalene. Both PAC and RRR operate efficiently at high temperatures leading through rapid molecular mass growth processes to complex aromatic structures, which are difficult to synthesize via traditional pathways such as Hydrogen‐Abstraction/Acetylene‐Addition.
Contact
Ralf I. Kaiser, Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822, USA. Email: ralfk@hawaii.edu
Alexander M. Mebel, Department of Chemistry and Biochemistry, Florida International University, Miami, FL 33199, USA. Email: mebela@fiu.edu
Musahid Ahmed, Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA. Email: mahmed@lbl.gov
Funding
This work was supported by the US Department of Energy, Basic Energy Sciences.
Publications
L. Zhao, M. Prendergast, R.I. Kaiser, B. Xu, M. Ahmed, B.J. Sun, Y.L. Chen, A.H.H. Chang, R. Mohamed, F.R. Fischer, “Synthesis of Polycyclic Aromatic Hydrocarbons via Phenyl Addition - Dehydrocyclization: The Third Way.”Angew Chem Int Ed. (2019) [DOI: 10.1002/anie.201909876]
L. Zhao, R.I. Kaiser, W. Lu, B. Xu, M. Ahmed, A.N. Morozov, A.M. Mebel, A.H. Howlader, S.F. Wnuk, “Molecular mass growth through ring expansion in polycyclic aromatic hydrocarbons via radical–radical reactions.” Nature Communications 10, 3689 (2019). [DOI: 10.1038/s41467-019-11652-5]
Related Links
Lawrence Berkeley National Laboratory press release: Study Reveals ‘Radical’ Wrinkle in Forming Complex Carbon Molecules in Space