Water: Lead, Follow, or Get Out of the Way
Elegant theory shows how water helps separate ions involved in material synthesis and manufacturing.
The Science
Inside fuel cells, batteries, and biological systems, pairs of ions in water can affect chemical reactions. Knowing more about how water influences those reactions could be helpful. Theorists designed a simple, elegant method that explains the influence. Their method shows how water moves around ions and causes them to draw together or stay apart.
The Impact
Ion pairing can be an important factor in chemical and biological processes. Joining ions involves complex motions of networks made of water molecules. The team’s approach offers insights into ions and how they pair. The results will let scientists predict, control, and tune structure, function, and dynamics of ions and related processes.
Summary
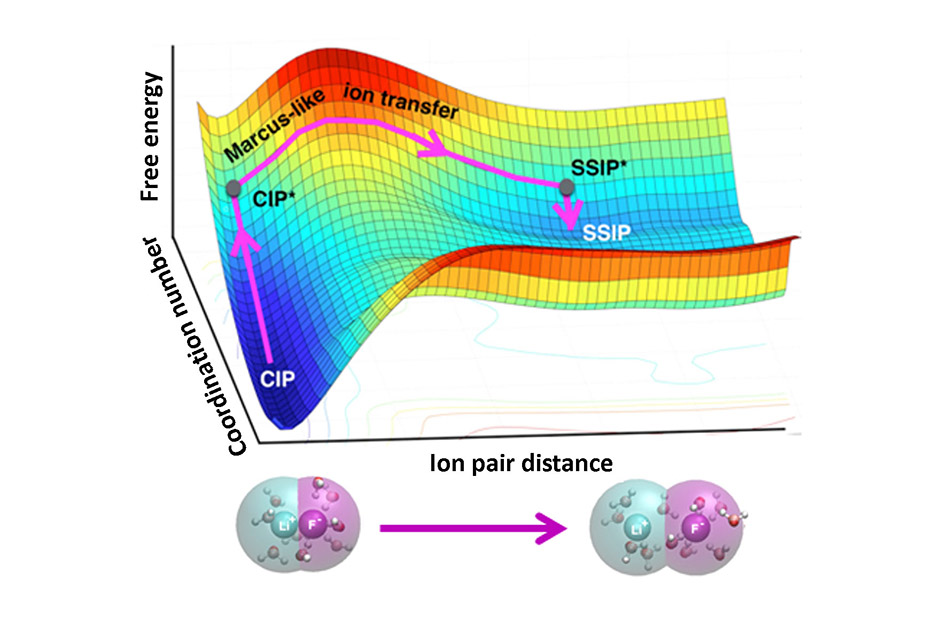
The association or dissociation of ion pairs in water is present in chemical reactions in fuel cells, batteries, and human cells. Scientists have struggled to understand how water molecules that cluster around the ions influence the reactions. The challenge? Traditional computational models often contain too much information to reveal the desired features. A team of theorists designed a simple, elegant method that shows how water moves around pairs of ions and influences whether they draw together or stay apart. To develop the theory for ion pairs, the team explored the distance between the ions and the number of water molecules around either the individual ion or the ion pair.
Applying the approach, the team revealed that ion dissociation occurs in two stages. First, there is an increase in the number of water molecules around each ion. Second, the ions move apart. For ions to join together, water molecules must move out of the way. Water moving is the critical, rate-limiting step. The team’s framework draws from Marcus theory, originally designed to calculate how fast electrons transfer between molecules in solutions and later extended to other transformations. The team’s method offers an improved understanding of ion pairs that will let researchers control and tune structure, function, and dynamics of ion pairs in different systems, from proteins’ interactions with DNA to ions’ motion in batteries.
Contact
Gregory Schenter
Pacific Northwest National Laboratory
greg.schenter@pnnl.gov
Funding
S.R., C.J.M., and G.K.S. were supported by the Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division. M.D.B. was supported by Materials Synthesis and Simulation Across Scales Initiative, a Laboratory Directed Research and Development program at Pacific Northwest National Laboratory (PNNL). The research was performed using PNNL Institutional Computing.
Publications
S. Roy, M.D. Baer, C.J. Mundy, and G.K. Schenter, “Marcus theory of ion-pairing.” Journal of Chemical Theory and Computation 13, 3470 (2017). [DOI: 10.1021/acs.jctc.7b00332]
Highlight Categories
Performer: DOE Laboratory