Hybrid Material Glows Like Jellyfish
Scientists combine biology, nanotechnology into composites that light up upon chemical stimulation.
The Science
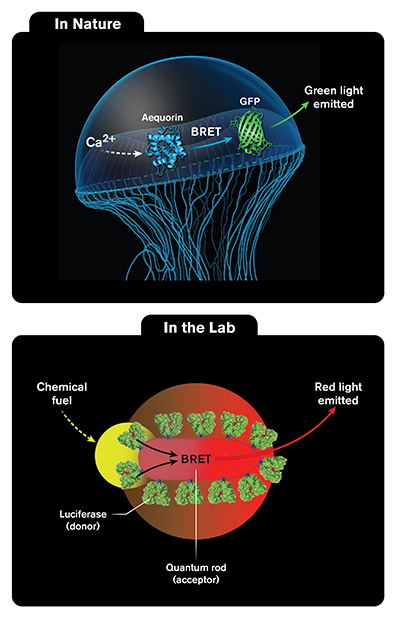
Jellyfish get their beautiful, greenish-blue glow from a chemical reaction. Inspired by this natural process, scientists from Syracuse University designed and built a material that mimics the process. The material combines tiny, synthetic rods and natural enzymes. Combined correctly, these materials yield light. That is, the material glows red when chemically stimulated. It follows a process similar to that in jellyfish.
The Impact
The material produces light without batteries or wall plugs. It shows how to control a bioluminescence process. Detailed analysis of the material offered insight into turning chemical energy into light more efficiently.
Summary
Improving the efficiency of nanoscale energy transfer in quantum dot or quantum rod based systems requires the ability to fine tune variables influencing this process, such as the distance between donor and acceptor, stoichiometry, and donor and acceptor spectral overlap. In most energy transfer cases, quantum dots or rods are used as donors because they efficiently harvest light with broad absorption extending to ultraviolet wavelengths. In this study, scientists created a nanohybrid biotic-abiotic system inspired by nature’s bioluminescent energy transfer (BRET) process. In BRET, the donor is a biomolecule that undergoes chemical stimulation, leading to bioluminescence, which is then transferred to an acceptor biomolecule that emits light. Scientists chose as the donor a luciferase enzyme that emits green light following chemical stimulation and the acceptor as a core-shell nanocrystal designed to absorb and emit in the red spectral region. The results allowed the research team to elucidate the design parameters needed to improve BRET efficiency and to understand the underlying BRET mechanism by the use of a biotic-abiotic nanohybrid system.
Contact
Mathew M. Maye
Department of Chemistry, Syracuse University
mmmaye@syr.edu
Grace Webster
Center for Functional Nanomaterials, Brookhaven National Laboratory
gwebster@bnl.gov
Funding
This research was supported by the Air Force Office of Scientific Research. This research used resources of the Center for Functional Nanomaterials, which is a U.S. Department of Energy Office of Science user facility, at Brookhaven National Laboratory.
Publications
R. Alam, L.M. Karam, T.L. Doane, K. Coopersmith, D.M. Fontaine, B.R. Branchini, and M.M. Maye, “Probing bioluminescence resonance energy transfer in quantum rod–luciferase nanoconjugates.”ACS Nano 10, 1969 (2016). [DOI: 10.1021/acsnano.5b05966]
Related Links
Phys.org article: Syracuse chemists combine biology, nanotechnology to create alternate energy source
Highlight Categories
Performer: University , SC User Facilities , BES User Facilities , CFN
Additional: Collaborations , Non-DOE Interagency Collaboration