Stacking Metals for Better Catalysis: A New Type of Metal-Support Interaction
Designing a novel catalyst for the production of hydrogen.
The Science
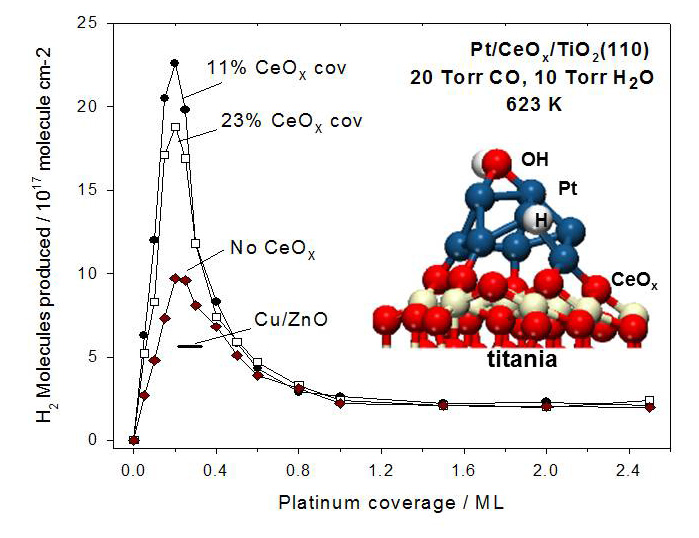
Combining experimental and theoretical results, this study showed a new type of “strong metal-support interaction” containing ceria (CeO2) along with titania (TiO2) that modifies the electronic properties of platinum (Pt) nanoparticles enhancing water cleavage into hydrogen by 20-fold, well beyond known effects.
The Impact
Developing improved solid catalysts, electrocatalysts, and photocatalysts is essential to improving the efficiency of current fuel and photovoltaic cells. This new type of “strong metal-support interaction” can be used to design novel catalysts that are highly active for the production of hydrogen, a necessary component for chemical processes and technological applications.
Summary
BES-supported researchers at Brookhaven National Laboratory discovered a new type of metal-support interaction useful for the development of more efficient catalysts. Catalysts are often "supported," meaning the most active component, usually a noble metal, is dispersed as nanoparticles or thin layers over a high surface-area material (usually an oxide) to improve catalyst effectiveness by exposing more of the active component to the reacting fluid. The main function of the support is to spread out the active component and prevent its aggregation or clustering which lower overall activity. In addition, the support possesses chemical functions on its surface and at the interface with the active components that contribute strongly to the catalytic reaction. In heterogeneous catalysis, the Pt/TiO2 system can be affected by “strong metal-support interactions,” which usually deactivate platinum possibly due to small aggregates of TiO2 decorating the surface of the Pt particles. However, this study observed a completely different phenomenon. The results of valence photo-emission and density functional calculations point to a new type of “strong metal-support interaction” which produces large electronic perturbations for small Pt particles in contact with ceria and significantly enhances the ability of Pt to produce hydrogen by dissociating the O-H bonds in water. This catalytic activity of Pt increases further when TiO2 is introduced to support the Pt/CeOx catalyst. This new type of “strong metal-support interactions” could play an important role when designing metal/oxide catalysts containing Pt to increase overall catalytic activity.
Contact
Jose A Rodriguez
Chemistry Department Brookhaven National Laboratory
rodrigez@bnl.gov
(631) 344-2246
Funding
DOE Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division.
Publications
Albert Bruix, José A. Rodriguez, Pedro J. Ramírez, Sanjaya D. Senanayake, Jaime Evans, Joon B. Park, Dario Stacchiola, Ping Liu, Jan Hrbek and Frances Illas “A New type of Strong Metal-Support Interaction and H2 Production through the Transformation of Water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) Catalysts”, Journal of the American Chemical Society, 2012, 134, 8968-8974. [DOI: 10.1021/ja302070k]
Related Links
Nature Chemistry News and Views (subscription required)
Highlight Categories
Performer: DOE Laboratory
Additional: Collaborations , International Collaboration