Turning Down the Heat on Carbon
Unusual reaction eschews high temperatures and water to lock away climate-changing carbon dioxide.
The Science
A reaction has been discovered that requires relatively low temperatures and recycles water as it turns carbon dioxide into a solid mineral, trapping the greenhouse gas away from the atmosphere.
The Impact
Provides new insights on a reaction that transforms carbon dioxide into a solid mineral, an important reaction as scientists look to sequester carbon underground.
Summary
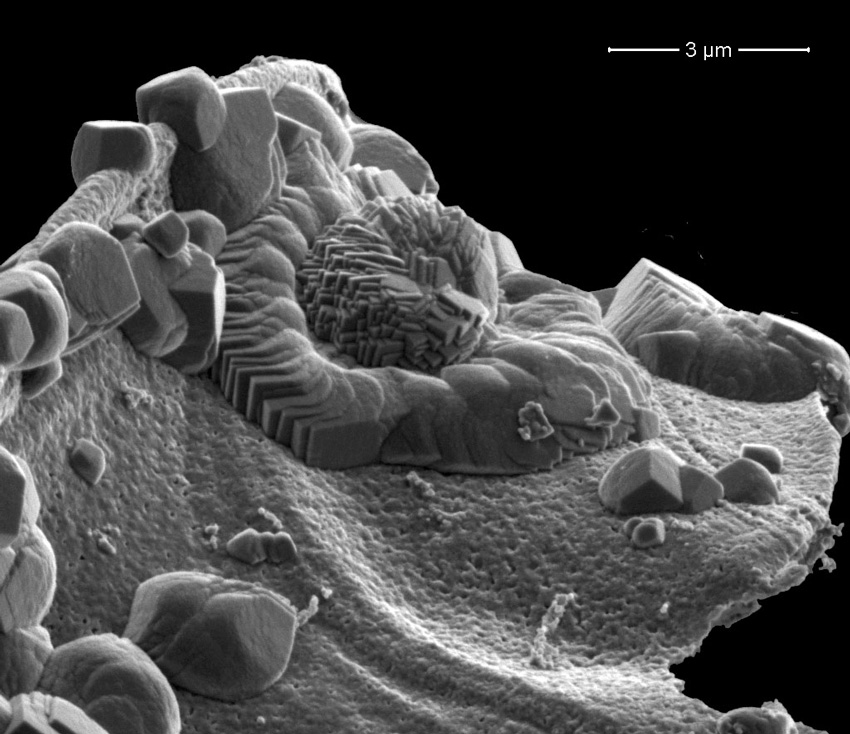
Keeping power plants' emissions of carbon dioxide sequestered underground, even in the event of an earthquake or other geological upset, will be facilitated by transforming the carbon dioxide into a mineral, such as anhydrous magnesite. This reaction occurs more readily at high temperatures and requires additional water – conditions that may not be viable in an underground reservoir. Scientists at Pacific Northwest National Laboratory discovered a reaction that forms the desired mineral at the relatively low temperature of 95°F and while recycling the water it needs. The team began with highly reactive nanometer-sized particles of an abundant mineral called forsterite, MgSiO4. They introduced water-saturated supercritical carbon dioxide to the particles. They examined the forsterite surface using scanning electron microscopy and characterized the molecules formed using four specialized spectrometers. The results show that within 3 to 4 days, the forsterite, water, and carbon dioxide form a mixture of two magnesium-based minerals: nesquehonite, which contains water, and the desired anhydrous magnesite, which does not. Water is continually used and released in the process, with the water driving the reaction. Over 14 days of reacting, the carbon dioxide was transformed into magnesite and a highly porous silica phase.
Contact
Andrew Felmy
ar.felmy@pnnl.gov
Kevin Rosso
kevin.rosso@pnnl.gov
Funding
DOE Office of Science Basic Energy Sciences (BES); research performed at the Environmental Molecular Sciences Laboratory, an Office of Science user facility managed by the Office of Biological and Environmental Research (BER).
Publications
Felmy AR et al. "Reaction of Water-Saturated Supercritical CO2 with Forsterite: Evidence for Magnesite Formation at Low Temperatures."Geochimica et Cosmochimica Acta 91 271-282 (2012). [DOI: 10.1016/j.gca.2012.05.026.]
Related Links
http://www.pnl.gov/science/highlights/highlight.asp?id=1187
Highlight Categories
Performer: DOE Laboratory , SC User Facilities , BER User Facilities , EMSL