Mimicking Photosynthesis for Production of Solar Fuels
A step closer to an artificial system using sunlight to produce hydrogen from water
The Science
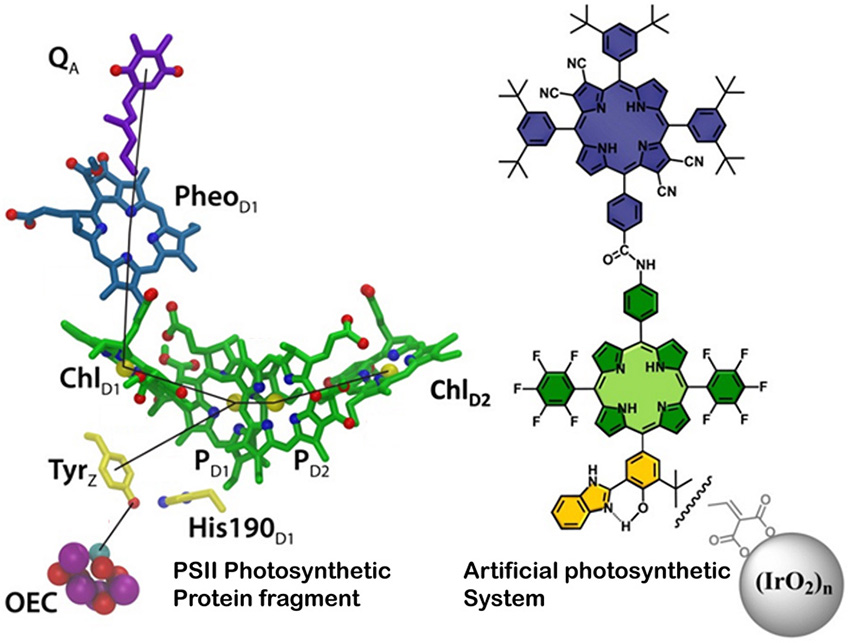
Using nature to inspire design, EFRC researchers constructed an artificial photosynthetic apparatus that uses sunlight to break apart water into oxygen and hydrogen fuel.
The Impact
This basic science discovery using sunlight to make renewable hydrogen is a step towards energy independence through development of solar fuel systems. Such systems also have the potential to reduce the environmental impacts of human energy use.
Summary
In natural photosynthesis, multiprotein complexes termed photosystems capture solar energy and convert it to chemical energy. Sunlight is absorbed by a pigment in the photosynthetic reaction center of the photosystem, causing electrons to increase in energy. These high-energy electrons are then transported through a series of components to the water splitting/oxygen evolving complex in the photosystem where via the energy from the electrons, water molecules are split into oxygen and hydrogen ions. Using natural photosynthesis as a model, The Center for Bio-Inspired Solar Fuel Production EFRC at Arizona State University designed a completely artificial photosynthetic fuel production system. Researchers constructed synthetic versions of each of the key parts of electron absorption and electron transfer found in a natural photosystem. Ultrafast laser studies verified that the synthetic version functioned in a manner similar to the natural version. The water splitting/oxygen evolving component was added through collaboration with Pennsylvania State University. When illuminated, the final completed solar water splitting device produces oxygen and hydrogen gas from water. While this artificial system is inefficient and currently operated only on the laboratory scale, it is a step forward in finding a way to a viable solar fuel technology.
Contact
Devens Gust
Arizona State University, Director
Center for Bio-Inspired Solar Fuel Production EFRC
gust@asu.edu
Thomas E. Mallouk
Pennsylvania State University
tem5@psu.edu
Funding
DOE Office of Science, Office of Basic Energy Sciences, Energy Frontier Research Centers (EFRC) Program (PSII mimic and phenol-bisimidazole) and Chemical Sciences, Geosciences, and Biosciences Division (iridium oxide cell preparation and characterization)
Publications
Megiatto, J. D. Jr.; Antoniuk-Pablant, A.; Sherman, B. D.; Kodis, G.; Gervaldo, M.; Moore, T. A.; Moore, A. L.; and Gust, D. “Mimicking the electron transfer chain in photosystem II with a molecular triad thermodynamically capable of water oxidation,” Proc. Natl. Acad. Sci. U. S. A., 109,15578 (2012). [DOI: 10.1073/pnas.1118348109].
Zhao, Y.; Swierk, J. R.; Megiatto, J. D. Jr., Sherman, B.; Youngblood, W. J.; Qin, D.; Lentz, D. M.; Moore, A. L.; Moore, T. A.; Gust, D.; and Mallouk, T. A. “Improving the efficiency of water splitting in dye-sensitized solar cells by using a biomimetic electron transfer mediator,” Proc. Natl. Acad. Sci. U. S. A., 109, 15612-15616, (2012). [DOI: 10.1073/pnas.1118339109]
Related Links
Center for Bio-Inspired Solar Fuel Production (BISfuel) EFRC
Highlight Categories
Performer: University