New Catalyst Opens Way to Next-Generation Fuel Cells
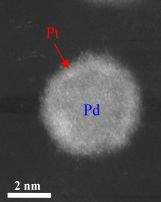 Image courtesy of Brookhaven National Laboratory.
Image courtesy of Brookhaven National Laboratory.
This high-angle annular dark field (HAADF) scanning transmission electron microscopy (STEM) image shows a bright shell on a relatively darker nanoparticle, signifying the formation of a core/shell structure — a platinum monolayer shell on a palladium nanoparticle core.
Stop-and-go driving can wear on your nerves, but it really does a number on the precious platinum that drives reactions in automotive fuel cells. Before large fleets of fuel-cell-powered vehicles can hit the road, scientists will have to find a way to protect the platinum, the most expensive component of fuel-cell technology, and to reduce the amount needed to make catalytically active electrodes.
Scientists at the U.S. Department of Energy's Brookhaven National Laboratory, in collaboration with Toyota Motor Corporation, are closing in on that goal. The researchers developed a new electrocatalyst that uses a single layer of platinum and minimizes its wear and tear while maintaining high levels of reactivity during tests that mimic stop-and-go driving. The research may greatly enhance the practicality of fuel-cell vehicles and may also be applicable for improving the performance of other metallic catalysts.
"Fuel cells are expected to become a major source of clean energy that can impact both transportation and stationary power sectors," said Brookhaven Lab chemist Radoslav Adzic, who leads the research team. "They have several advantages for automotive applications and can be used extensively in electric cars if the technology can be made to work efficiently and economically. Developing these electrocatalysts is a big step in that direction."
 Image courtesy of Brookhaven National Laboratory.
Image courtesy of Brookhaven National Laboratory.
(From left) Brookhaven Lab chemists Kotaro Sasaki, Miomir Branko Vukmirovic, and Radoslav Adzic work on developing catalysts for fuel cells at the National Synchrotron Light Source.
The newly designed fuel cell catalysts are composed of a single layer of platinum over a palladium (or palladium-gold alloy) nanoparticle core. Their structural characterization was performed at Brookhaven's Center for Functional Nanomaterials and the National Synchrotron Light Source, both national scientific user facilities supported by the DOE Office of Science.
Fuel cells converts hydrogen and oxygen into water and, as part of the process, produce electricity. Hydrogen is oxidized when electrons are released and hydrogen ions are formed; the released electrons supply current for an electric motor. Oxygen is reduced by gaining electrons, and in a reaction with hydrogen ions, water—the only byproduct of a fuel cell reaction—is produced.
Platinum electrocatalysts are used to speed up the oxidation and reduction reactions involved in this process, but as a result, they, too, are oxidized (lose electrons) and dissolve. Over time, as oxidation and reduction reactions cycle—triggered by changes in voltage that occur during stop-and-go driving—the platinum is damaged, eventually destroying the fuel cell.
In the new catalyst, palladium from the core is more reactive than platinum in these oxidation and reduction reactions. Stability tests simulating fuel cell voltage cycling revealed that, after 100,000 potential cycles, a significant amount of palladium had been oxidized, dissolved, and migrated away from the cathode. In the membrane between the cathode and anode, the dissolved palladium ions were reduced by hydrogen diffusing from the anode to form a "band," or dots.
“Fuel cells are expected to become a major source of clean energy that can impact both transportation and stationary power sectors.”
In contrast, platinum was almost unaffected, except for a small contraction of the platinum monolayer. This contraction of the platinum lattice makes the catalyst more active and the stability of the particles increases.
Reactivity of the platinum monolayer/palladium core catalyst also remained extremely high. It was reduced by merely 37 percent after 100,000 cycles.
"Our studies of the structure and activity of this catalyst—and comparisons with platinum-carbon catalysts currently in use—illustrate that the palladium core 'protects' the fine layer of platinum surrounding the particles, enabling it to maintain reactivity for a much longer period of time," Adzic said.
Building on earlier work that illustrated how small amounts of gold can enhance catalytic activity, the scientists also developed a form of the platinum monolayer catalyst with a palladium-gold alloy core. The addition of gold further increased the stability of the electrocatalyst, which retained nearly 70 percent of reactivity after 200,000 cycles of testing.

Researcher Jia Wang at the Center for Functional Nanomaterials
"This indicates the excellent durability of this electrocatalyst, especially when compared with simpler platinum-carbon catalysts, which lose nearly 70 percent of their reactivity after much shorter cycling times. This level of activity and stability indicates that this is a practical catalyst. It exceeds the goal set by DOE for 2010-2015, and it can be used for automotive applications."
Fuel cells made using the new catalyst would require only about 10 grams of platinum per car—and less than 20 grams of palladium, Adzic said. Currently, in catalytic convertors used to treat exhaust gases, 5 to 10 grams of platinum is used. Since fuel-cell-powered cars would emit no exhaust gases, there would be no need for such catalytic converters, and therefore no net increase in the amount of platinum used.
"In addition to developing electrocatalysts for automotive fuel cell applications, these findings indicate the broad applicability of platinum monolayer catalysts and the possibility of extending this concept to catalysts based on other noble metals," Adzic said.
—Kendra Snyder, Brookhaven National Laboratory, ksnyder@bnl.gov
Funding
Basic Research: DOE Office of Science, Office of Basic Energy Sciences
Scientific User Facilities (National Synchrotron Light Source and Center for Functional Nanomaterials): DOE Office of Science, Office of Basic Energy Sciences
Follow-on Applied R&D: DOE Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Program; and Toyota Motor Corporation
Publication
K. Sasaki et al. "Core-Protected Platinum Monolayer Shell High-Stability Electrocatalysts for Fuel-Cell Cathodes," Angewandte Chemie Int. Ed. 49 8602 (2010).
Link: http://onlinelibrary.wiley.com/doi/10.1002/anie.201004287/abstract
Related Links
Brookhaven Lab Chemists Receive Patents for Fuel-Cell Catalysts
(http://www.bnl.gov/bnlweb/pubaf/pr/PR_display.asp?prID=1134)
Brookhaven Lab Scientists Discover Gold Clusters Stabilize Platinum Electrocatalysts for Use in Fuel Cells
(http://www.bnl.gov/bnlweb/pubaf/pr/PR_display.asp?prID=07-04)

