Graphene: Amazing Material Found Thanks to Scotch Tape and Persistent Science
That's the developing story of graphene, an amazing material that can be found in pencil lead. Pencil "lead" is actually a mixture of clay and the material graphite. Graphite itself is composed entirely of the element carbon, and consists of stacked layers of graphene. Graphene is a one carbon atom thick layer with the atoms arranged in a honeycomb structure, and its properties are extraordinarily different from graphite.
Scientists had previously discovered single-layered carbon structures, such as rolled up sheets of carbon known as nanotubes and hollow balls of carbon commonly called fullerenes or buckeyballs. But few believed that single sheets of carbon could be produced: They were thought to be too unstable.
That's where Andre Geim and Konstantin Novoselov came in. They took a hunk of graphite and used Scotch tape to peel off layer after layer after layer. Geim and Novoselov then analyzed what they had left, and found graphene. For their discovery – which was published in 2004 – they were awarded the 2010 Nobel Prize in Physics.
Graphene research accelerated in 2005, when further research by Geim and his team as well as a group led by Philip Kim, demonstrated that graphene's electrons behave in a relativistic way, which causes the material to act as a cross between a metal and a semiconductor. Kim, a physics professor at Columbia University supported by DOE's Office of Science, has made many advances in the field. As Geim noted of Kim, "He made an important contribution, and I would gladly have shared the [Nobel] prize with him."
Graphene has been observed to be more than 100 times stronger than steel – so strong that a thin layer could support an elephant. It's also stretchable and almost transparent– and a slightly better electrical conductor than copper. It is expected that transistors made from graphene could be significantly faster than today's silicon chips. Electrons in graphene have some unique characteristics, as noted by Boston University physics professor Antonio Castro Neto, who is studying graphene, also with support from the DOE Office of Science. These electrons can be described using the relativistic wave equation. (Materials Today, march 20, vol 13#3).
Since its discovery, researchers have continued to analyze the graphene's unique properties and are working to bring about its use in a wide array of applications and potential products. For instance, researchers at the Center for Nanoscale Materials at Argonne National Laboratory are using the highest resolution microscopes to further understand graphene's structural and electrical properties. Scientists at Center for Nanophase Materials Sciences at Oak Ridge National Laboratory have discovered how to minimize the loops that can form in graphene's structure, which interfere with its unique electrical properties:
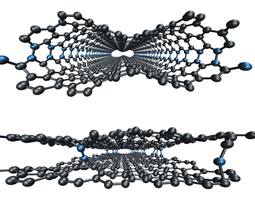
Oak Ridge National Laboratory
ORNL simulations demonstrate how loops (seen above in blue) between graphene layers can be minimized using electron irradiation (bottom).
Researchers at University of California, Los Angeles, and the Molecular Foundry, Lawrence Berkeley National Laboratory, are studying the "noise" in thin strips of graphene known as "nanoribbons" which will likely be useful in building more efficient computer chips. And scientists at Pacific Northwest National Laboratory's Environmental Molecular Sciences Laboratory and Princeton University have teamed up to tie graphene to single strands of DNA, which could lead to better, more stable biosensors to diagnose diseases.
Those are just the first glimpses of what is likely to be a whole world of astonishing uses. In addition to faster computers and better sensors, graphene could also be used in transparent touch screens and better solar cells, safer cars and lighter satellites. More possibilities will certainly be discovered as scientists learn more about the material and become better at producing it in larger scales and more cost-efficient fashion.
We're on the cusp of seeing graphene's amazing properties at work in a world of new ways. We're getting closer thanks to the work supported by the DOE Office of Science. And it all started with pencil lead, Scotch tape, and a few persistent scientists.
For more information on graphene research at our National Labs go to Argonne, Lawrence Berkeley, Oak Ridge and Pacific Northwest.
For more information on graphene and the Nobel Prize, go to: http://static.nobelprize.org/nobel_prizes/physics/laureates/2010/info_publ_phy_10_en.pdf or http://static.nobelprize.org/nobel_prizes/physics/laureates/2010/sciback_phy_10.pdf
Charles Rousseaux is a Senior Writer in the Office of Science.