Studying the Mechanism of Metal Extraction with Ionic Liquids
Research on techniques for studying the chemical properties of superheavy elements might also help recover a strategically important metal.
The Science
The heaviest known elements are the so-called “superheavy” elements, those with atomic numbers greater than 103. These elements are found only in laboratories, where they are made by fusing together two lighter elements. This process is unlikely to occur, so scientist have only tiny amounts (a few atoms) for experiments. Chemists are interested in the chemical properties of these elements. However, the small amounts of material available means chemists must use special techniques to study them. This research developed a new way to study the chemistry of metallic elements with extremely low concentrations of material. These techniques use ionic liquids—salts in liquid states.
The Impact
This new extraction technique can help probe the chemistry of superheavy elements. For example, scientists know almost nothing about the superheavy element nihonium. Learning more about nihonium can help scientists study elements like indium and thallium. These two elements are not among the superheavy elements, but scientists believe they are homologs (chemically similar elements) of nihonium. This understanding may lead to better methods of recovering iridium, an element that is critical to national security and the economy. Because iridium is not currently mined in the United States, improved recovery methods are a potential benefit to the country.
Summary
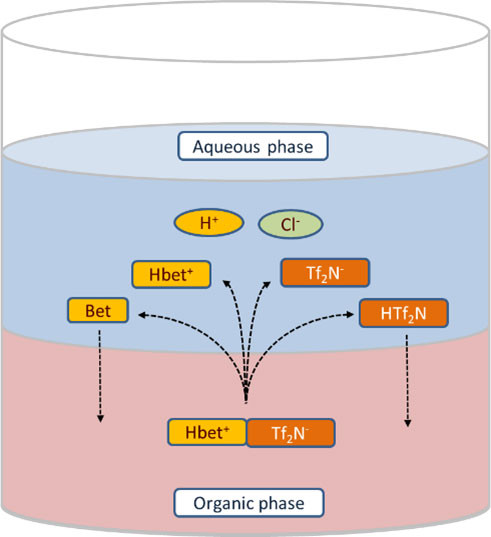
Ionic liquids are organic solvents made entirely of ions, and they are very different from traditional organic solvents. In this research, scientists studied the ionic liquid betainium bis(trifluoromethylsulfonyl)imide for its ability to extract indium and thallium from solutions of hydrochloric acid. In a first study, scientists examined the interaction of the ionic liquid with water for a range of concentrations of hydrochloric acid and betaine. They determined the concentration of each species using quantitative nuclear magnetic resonance and titrations combined with a mathematical model. The researchers observed that the dissolution of betainium bis(trifluoromethylsulfonyl)imide in the aqueous phase initiated key chemical reactions. In a second study, researchers added both radioactive and non-radioactive isotopes indium and thallium to the aqueous phase, then measured their concentrations after extraction using gamma spectrometry and mass spectrometry. The researchers developed a new mathematical model to describe the data. This research offers a greater understanding of the extraction process for heavy elements by pointing to a potential method to recover indium, a strategically important metal. This research also identifies a potential method to study the chemical properties of the superheavy element nihonium.
Contact
Charles M. Folden III
Texas A&M University
Folden@comp.tamu.edu
Funding
Financial support was provided by the National Center for Scientific Research (CNRS, France) through its International Program for Scientific Cooperation (PICS). This material is based on work supported by the Department of Energy (DOE) Office of Science, Office of Nuclear Physics and by the DOE National Nuclear Security Administration through the Nuclear Science and Security Consortium.
Publications
Volia, M. F., Tereshatov, E. E., Boltoeva, M., and Folden, C. M. III, Indium and Thallium Extraction into Betainium Bis(trifluoromethylsulfonyl)imide from Aqueous Hydrochloric Acid Media. New Journal of Chemistry 44, 2527 (2020). [DOI 10.1039/C9NJ04879K]
Volia, M. F., Tereshatov, E. E., Mazan, V., Folden, C. M. III, and Boltoeva, M., Effect of Aqueous Hydrochloric Acid and Zwitterionic Betaine on the Mutual Solubility Between a Protic Betainium-Based Ionic Liquid and Water. Journal of Molecular Liquids 276, 296 (2019). [DOI: 10.1016/j.molliq.2018.11.136]
Highlight Categories
Performer: SC User Facilities , FES User Facilities , DIII-D