Improving Carbon Sequestration
Photo courtesy of Pacific Northwest National Laboratory
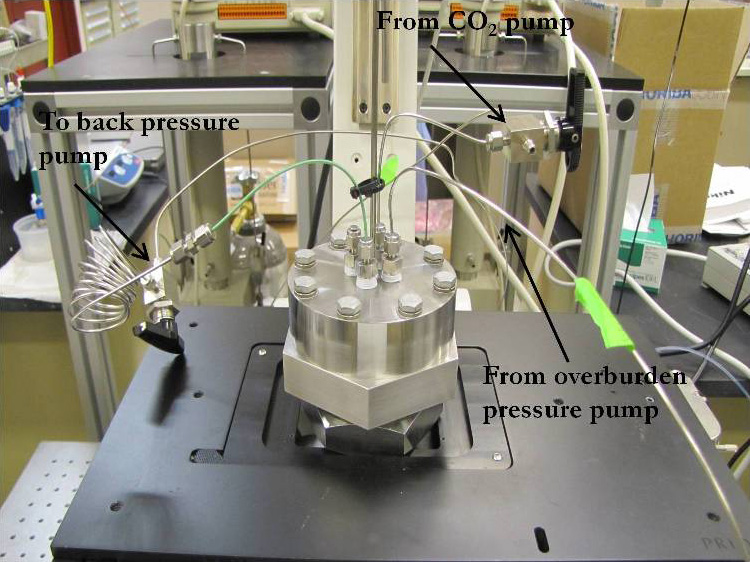
Supercritical CO2-brine displacement experimental setup for pressures up to 120 bar and 60°C.
Pacific Northwest National Laboratory (PNNL) researchers have developed a unique, high-pressure microfluidics system that enables pore-scale experimental studies of carbon dioxide (CO2) sequestration in aquifers or reservoirs. Scientists are using this unique system to study fluid-mineral interactions of CO2 under supercritical conditions (sc) in a range of geologic media. Scientific results are used to improve the carbon sequestration process, thereby reducing atmospheric CO2 levels and helping prevent CO2 re-emissions into the environment after sequestration.
Why it Matters
Geologic sequestration involves capturing and injecting scCO2 into a deep aquifer or reservoir for permanent or semi-permanent storage. Carbon dioxide usually behaves as a gas in air or as a solid in dry ice: if the temperature and pressure (greater than 73 atmospheres) are both increased, it transforms into scCO2 with properties midway between a gas and a liquid, making it unstable. Scientists are using the microfluidics system to study scCO2 sequestration. Studies are focused on addressing two technical challenges: potential leakage of scCO2 out of the reservoir into the caprock and subsequent release into the atmosphere and the storage capacity of the reservoir rock.
Methods
To address these scCO2 sequestration issues, the project team has designed and constructed a unique high-pressure microfluidics system for micromodel experimentation with the capability to obtain scientific data at supercritical conditions.
Because the system is portable, it can be coupled with several types of spectrometers to gather information on the chemical composition, structure, and electronic properties of water, minerals, and CO2 as they interact in real time. For example, a sample can be analyzed with ultraviolet-visible absorption spectroscopy, laser-induced time-resolved fluorescence spectroscopy, Fourier transform infrared spectrometry, Raman, and nonlinear optical spectroscopy. Without this system, each instrument would require expensive modifications to handle the high pressures and temperatures associated with geologic sequestration.
What’s Next
Understanding chemical and geologic reactions of scCO2 at the pore scale will help scientists improve the CO2 sequestration process and prevent leaks or gas emissions into the environment. The project team is conducting laboratory micromodel studies. Next steps include conducting more expansive field tests at a scCO2 sequestration site.
Acknowledgments
PNNL project team members include Mart Oostrom, Changyong Zhang, Jay Grate, Marvin Warner, and Tom Wietsma.
Publications
Grate JW, CY Zhang, TW Wietsma, MG Warner, NC Anheier Jr, BE Bernacki, G Orr, and M Oostrom. 2010. “A Note on the Visualization of Wetting Film Structures and a Nonwetting Immiscible Fluid in a Pore Network Micromodel Using a Solvatochromic Dye.” Water Resources Research 46:W11602.
Willingham TW, CY Zhang, CJ Werth, AJ Valocchi, M Oostrom, and TW Wietsma. 2009. “Using Dispersivity Values to Quantify the Effects of Pore-Scale Flow Focusing on Enhanced Reaction Along a Transverse Mixing Zone.” Advances in Water Resources 33(4):525–535.