Decoding the Lifecycle of Photogenerated Charges
Monitoring photo-excited electrons in real time with nanometer sensitivity reveals strengths and weaknesses in a common light-harvesting material.
The Science
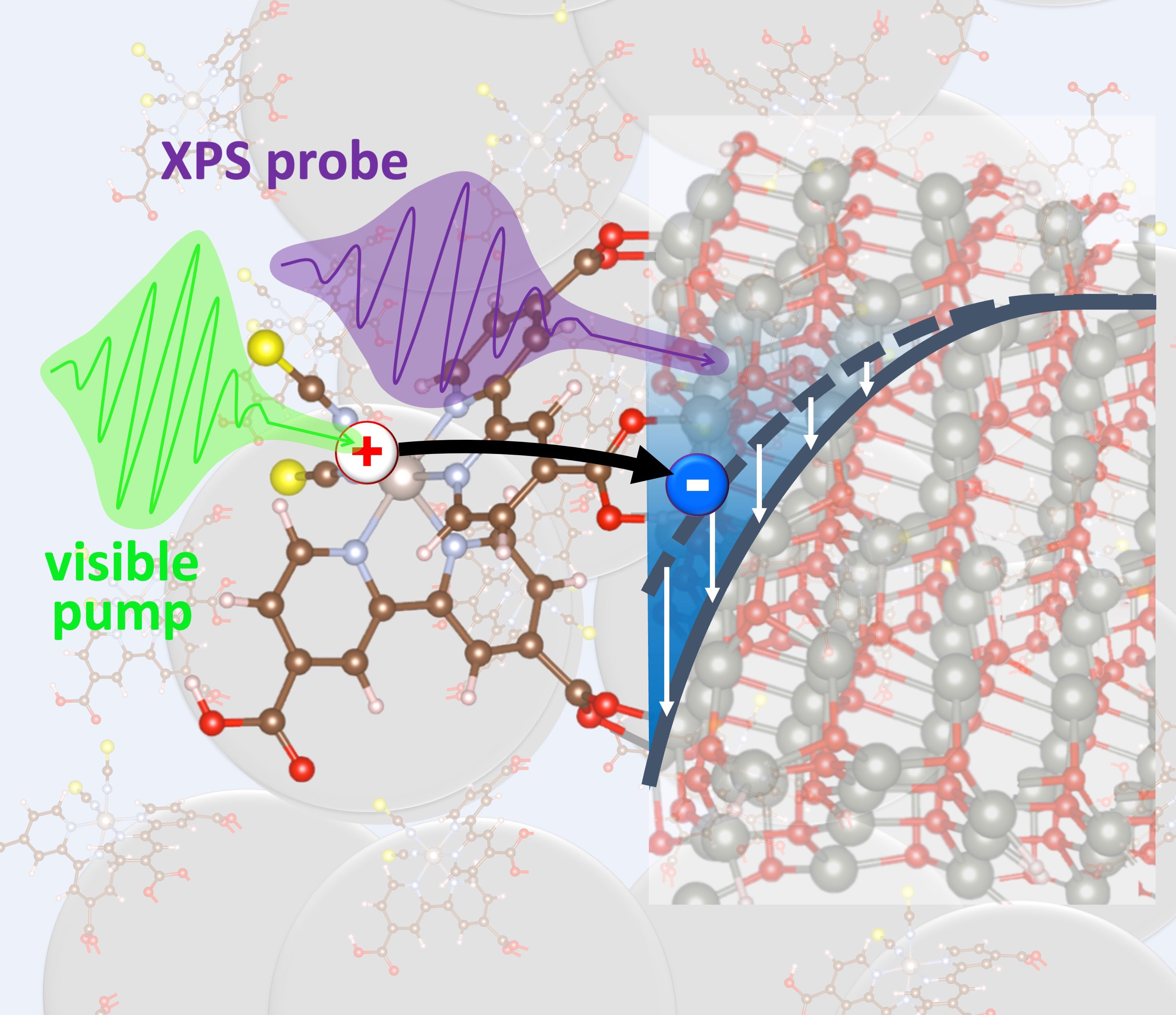
New materials will enable novel technologies to turn sunlight into electricity and fuels. Combinations of molecules and tiny nanoparticles make these materials a reality. The molecules in these materials are very good at absorbing sunlight and donating electrons to the nanoparticles. The nanoparticles then move the electrons around and catalyze reactions that produce the fuel. However, this process doesn't always work as researchers hope. Now scientists have found a way to track electrons along their round trip from the molecules to the nanoparticles and back. Researchers can measure where the electrons can travel easily and if, where, when, and why they get stuck. This information is crucial to finding better combinations for innovative materials.
The Impact
The study demonstrates a new experimental tool that can follow electrons traveling between molecules and nanoparticles that convert sunlight into electricity or fuels. It turns out that a very common nanoparticle material, zinc oxide, first stalls the electrons for a while. The material then lets the electrons move along only the very surface of the nanoparticles. This makes it likely that the charges can get lost or can damage the nanoparticle material. Ideally, charges should travel without pause and straight through the nanoparticles. The ability to reveal these bottlenecks for electron travel will help researchers design better materials for turning sunlight into other forms of energy.
Summary
To turn sunlight into electricity or fuel, a material must absorb the light and direct the light energy to electrons. Next, the electrons must move around to form a current or enable chemical reactions. One way to achieve both steps is to use molecules that are very good at catching sunlight and attach them to substrates that are very good at moving electrons around. Researchers previously knew that electrons could move around inside the material zinc oxide much more easily than in many other materials. Despite this fact, electrodes made of zinc oxide would not work as well as electrodes made of other materials. What is going on? Using a technique called time-resolved X-ray photoelectron spectroscopy at the Advanced Light Source, a Department of Energy (DOE) Office of Science user facility, researchers are now able to follow the path of the electrons from the molecules to the substrates and back. They found that the electrons are stuck for a long time between the molecules and the zinc oxide. When the electrons finally make the jump, the material keeps pushing them toward the substrate surface. There, the electrons get trapped more easily than if they were able to travel straight through the substrate bulk. This study helps explain why zinc oxide substrates don't work as well as hoped. It also provides a new testing scheme for future materials.
Contact
Oliver Gessner
Chemical Sciences Division
Lawrence Berkeley National Laboratory
OGessner@lbl.gov
Funding
This work was supported by the Atomic, Molecular, and Optical Sciences Program of the DOE Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division. Two of the researchers are supported by the Liquid Sunlight Alliance, a DOE Fuels from Sunlight Hub supported by the DOE Office of Science. The research used resources of the Advanced Light Source, a DOE Office of Science user facility. Some of the researchers also acknowledge support through the ALS Doctoral Fellowship in Residence Program, the Alexander von Humboldt Foundation and the Helmholtz Association, the Deutsche Forschungsgemeinschaft (e-conversion Cluster of Excellence), and the VI 419 of the Helmholtz Association.
Publications
Neppl, S., et al., Nanoscale Confinement of Photo-Injected Electrons at Hybrid Interfaces. Journal of Physical Chemistry Letters 12, 11951–11959 (2021). [DOI: 10.1021/acs.jpclett.1c02648]
Mahl, J., et al., Strong Potential Gradients and Electron Confinement in ZnO Nanoparticle Films: Implications for Charge-Carrier Transport and Photocatalysis. ACS Applied Nano Materials 4, 12213-12221 (2021). [DOI: 10.1021/acsanm.1c02730]
Highlight Categories
Performer: DOE Laboratory , SC User Facilities , BES User Facilities , ALS
Additional: Collaborations , International Collaboration