Placing Atoms for Optimum Catalysts
Precise positioning of oxygens could help engineer faster, more efficient energy-relevant chemical transformations.
The Science
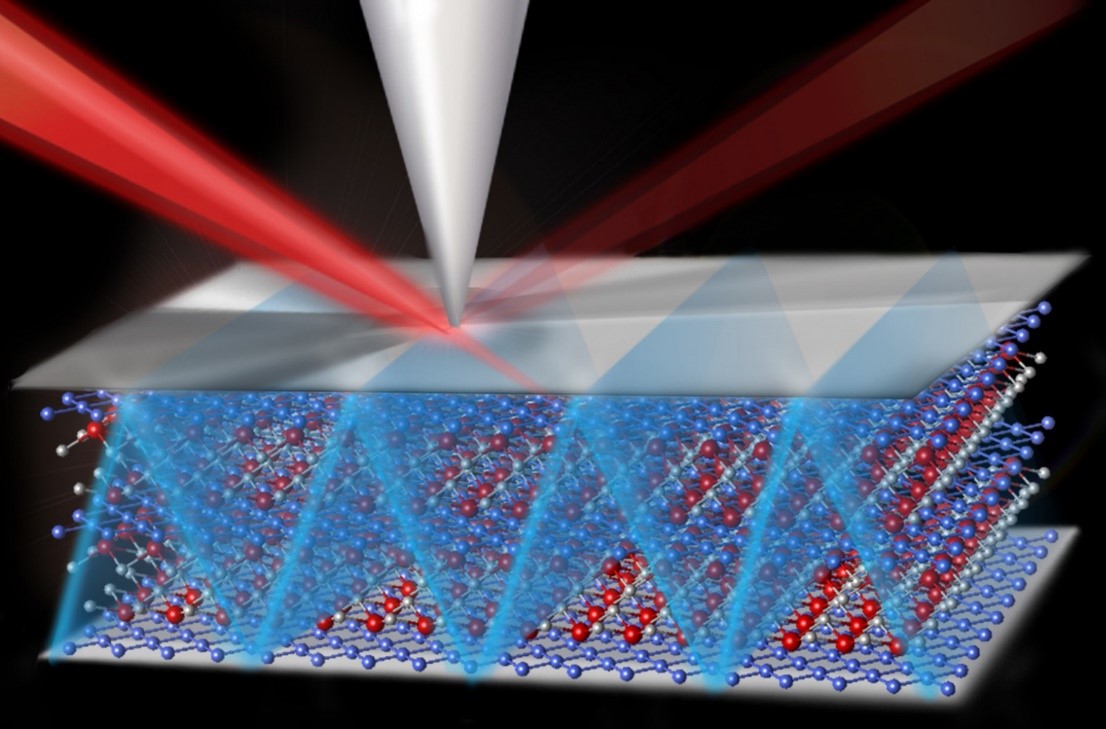
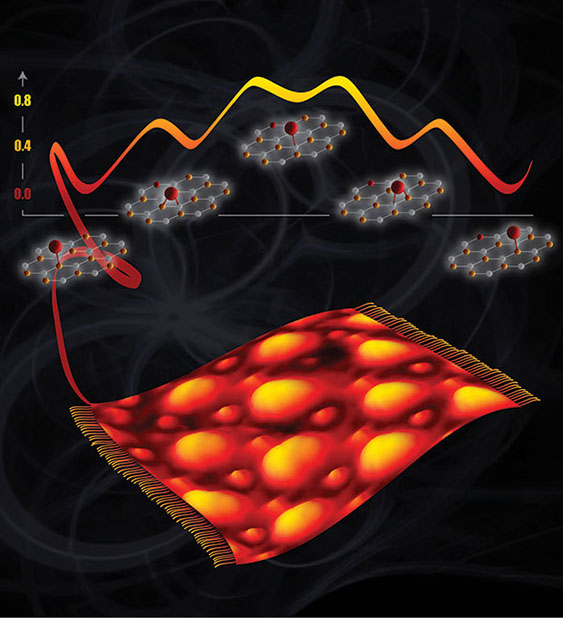
Fuels, plastics, and other products are made using catalysts, materials that drive chemical reactions. To design a better catalyst, scientists must get the right atoms in the right spot. Positioning the atoms can be difficult, but new research makes it easier. Researchers determined the exact location of single oxygen atoms, which act like anchors for catalysts. In the case of a layer of carbon atoms atop a metal support, single oxygen atoms appear in predictable spots. Knowing where the atomic anchors are, the team can create patterns of catalytic atoms, designing what’s needed to get the job done.
The Impact
Creating catalysts that make reactions faster and less wasteful means designing the catalysts from the bottom up. Rather than search among countless possibilities, scientists want to design the right structures at a molecular level. New fundamental research shows scientists how to take advantage of precise spots—where the oxygen atoms bind on graphene—to build model catalysts. This research redefines what is known about oxygen binding, which is vital to creating hard-working catalysts.
Summary
The team began with a flat piece of ruthenium metal. On top of the metal, they grew graphene, which is a one-atom-thick layer of carbon. In this structure, some carbon atoms bind to the metal, while others don't. By combining experimental and computational resources, the team examined these carbon atoms. They showed that single oxygen atoms, which act as ideal spots to attach catalytic sites, bind preferentially to carbon atoms that are close to the underlying metal but not bound to it. Less preferred sites for oxygen binding are between two carbon atoms; carbon atoms that are, in turn, bound to ruthenium; and untethered carbon atoms far from the ruthenium. This research redefines what scientists know about oxygen binding to carbon atoms on metal-supported graphene. The work is vital to designing efficient, selective catalysts.
Contact
Vassiliki-Alexandra Glezakou
Pacific Northwest National Laboratory
vanda.glezakou@pnnl.gov
Roger Rousseau
Pacific Northwest National Laboratory
roger.rousseau@pnnl.gov
Zdenek Dohnálek
Pacific Northwest National Laboratory
zdenek.dohnalek@pnnl.gov
Funding
The Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences; Alternate Sponsored Fellowship at Pacific Northwest National Laboratory (F.P.N.); and the University of Graz (F.P.N.) funded this research.
Publications
Z. Novotny, M.T. Nguyen, F.P. Netzer, V.A. Glezakou, R. Rousseau, and Z. Dohnálek, “Formation of supported graphene oxide: Evidence for enolate species.” Journal of the American Chemical Society 140(15), 5102 (2018). [DOI: 10.1021/jacs.7b12791]
Related Links
Pacific Northwest National Laboratory highlight: Tipping Water: Finding the Balance Between Keeping Molecules Whole or Splitting Them on Oxides
Highlight Categories
Performer: University , DOE Laboratory , SC User Facilities , ASCR User Facilities , NERSC , BER User Facilities , EMSL