Forever Young Catalyst Reduces Diesel Emissions
Atom probe tomography reveals key explanations for stable performance over a cutting-edge diesel-exhaust catalyst’s lifetime.
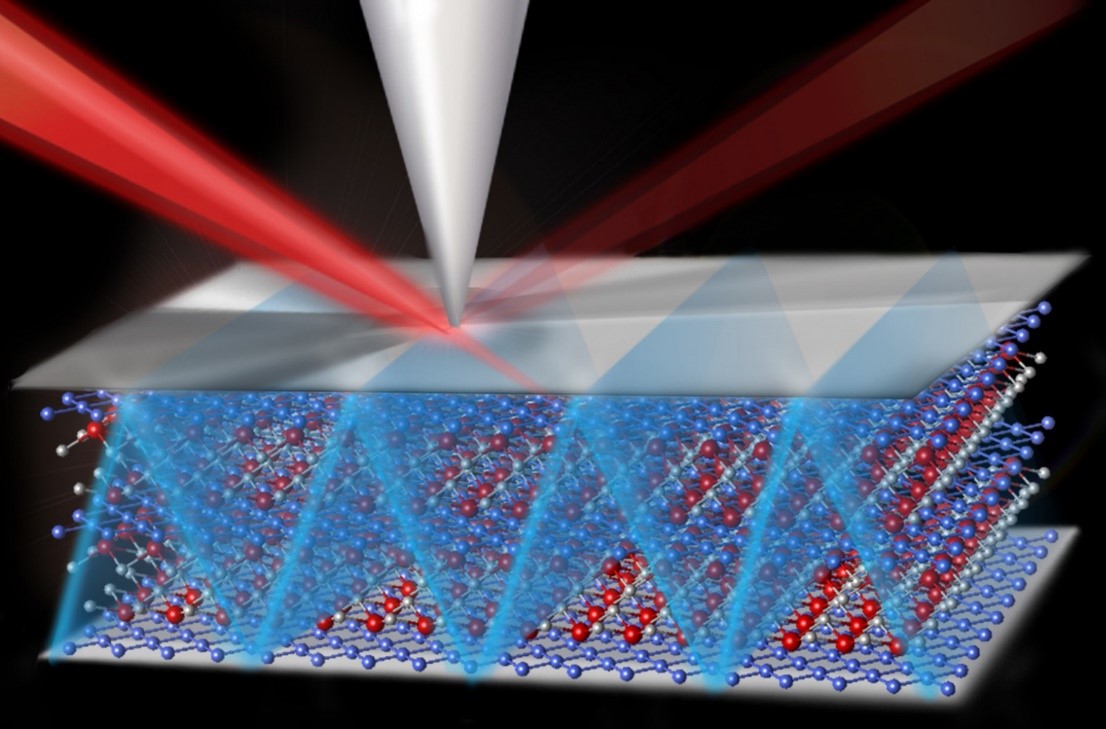
The Science
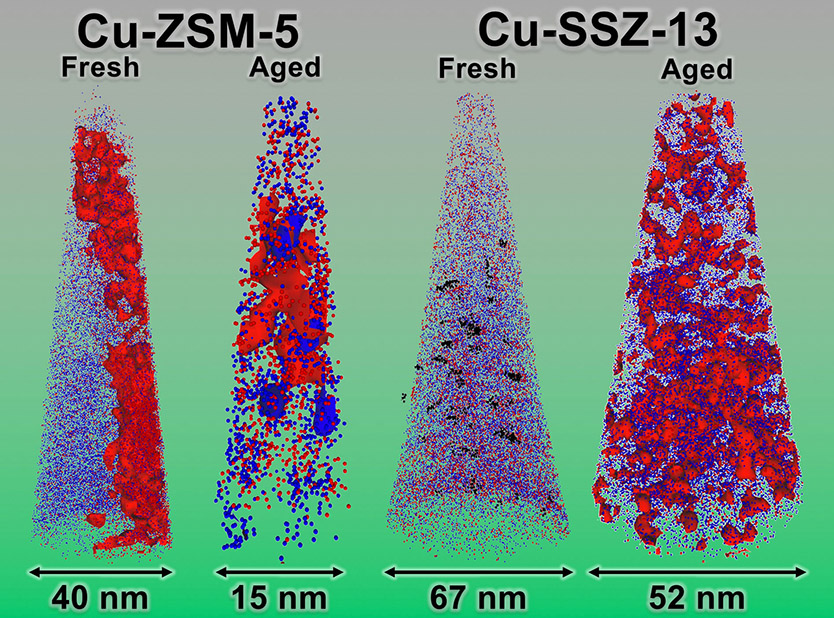
Diesel engines release pollutants. Specially designed catalysts reduce the amount of pollutants released. But what causes such catalysts to fail? Atom probe tomography provides the first direct observations of the problems. Scientists saw the atom-level redistributions that deactivate catalysts during simulated exposure to 135,000 miles of diesel exhaust. The team analyzed two copper-exchanged zeolite catalysts. The catalyst Cu-SSZ-13 has superior performance and stability compared to other commercial zeolite catalysts. The more common industrial catalyst, Cu-ZSM-5, can’t remain active for a vehicle’s lifetime. In this work, loss of catalytic activity correlates with the formation of tiny clusters. The clusters result from the collapse of the Cu-ZSM-5 framework and copper migration.
The Impact
Gaining a complete picture of the mechanism of catalyst deactivation through atom-by-atom 3-D reconstruction provides key information that the automotive industry needs to design better catalysts. These catalysts will perform as well at the end of a vehicle’s life as they did the day the car left the factory. Such performance is vital to keeping our air clean. In the chemical industry, where zeolite catalysts are broadly used, the knowledge gained may advance sustainable processes. These processes include biomass conversion, green plastics production, capture and conversion of carbon dioxide, remediation of air pollution and water purification.
Summary
Decreasing fuel consumption and emissions in diesel vehicles requires end-of-tailpipe technologies. To reduce emissions of nitrogen oxide pollutants that cause the formation of acid rain and smog, the most effective strategy is ammonia selective catalytic reduction using urea as the source of the ammonia reductant with the reaction occurring over a copper-exchanged zeolite catalyst. Such a catalyst is Cu-ZSM-5, which was originally described in 1986, but could not be fully commercialized as it deactivates well before the end of vehicle life. A major breakthrough in zeolite technology occurred in the mid-2000s when another copper-exchanged zeolite catalyst, Cu-SSZ-13, was discovered with unsurpassed activity and stability (that is,it cleans exhaust better over a longer period of time), and commercialization commenced quickly in 2010. Significant gaps remain in understanding the deactivation of zeolite catalysts, and thus, researchers at Utrecht University and Oak Ridge National Laboratory used APT to peer inside industry-relevant zeolites to visualize their nanoscale, elemental structure in 3-D. They looked at both Cu-ZSM-5 and Cu-SSZ-13 catalysts in fresh (that is, not exposed to diesel engine exhaust) and aged (simulated exposure to 135,000 miles of diesel exhaust) conditions. The study showed the nanoscale formation of a culprit phase causing catalytic deactivation (CuAl2O4l) through copper-aluminum aggregation, which was severe in Cu-ZSM-5. In contrast, Cu-SSZ-13 did not exhibit severe copper-aluminum aggregation nor evidence of the deactivating CuAl2O4 phase and was still able to optimally clean combustion products.
Contact
Jonathan Poplawsky
Center for Nanophase Materials Sciences, Oak Ridge National Laboratory
poplawskyjd@ornl.gov
Funding
This work is supported by the Netherlands Organisation for Scientific Research Gravitation program [of the Dutch government], Netherlands Center for Multiscale Catalytic Energy Conversion, and a European Research Council Advanced Grant. The atom probe tomography measurements were conducted at the Center for Nanophase Materials Sciences, a Department of Energy Office of Science user facility at Oak Ridge National Laboratory. Joel Schmidt received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant Agreement.
Publications
J.E. Schmidt, R. Oord, W. Guo, J.D. Poplawsky, and B.M. Weckhuysen, “Nanoscale tomography reveals the deactivation of automotive copper-exchanged zeolite catalysts.” Nature Communications 8, 1666 (2017). [DOI: 10.1038/s41467-017-01765-0]
Related Links
Utrecht University press release: Less diesel emissions thanks to catalyst converter that ‘stays young’
Oak Ridge National Laboratory feature: Researchers compare ‘new’ and ‘aged’ catalytic converter at the nanoscale level
Nanowerk article: Researchers compare ‘new’ and ‘aged’ catalytic converter at the nanoscale level
Phys.org article: Long-lived catalytic converter reduces diesel emissions
4-traders article: Universiteit Utrecht: Less diesel emissions thanks to catalyst converter that ‘stays young’
Public Now article: Less diesel emissions thanks to catalyst converter that ‘stays young’
Highlight Categories
Performer: University , DOE Laboratory , SC User Facilities , BES User Facilities , CNMS
Additional: Technology Impact , Collaborations , International Collaboration