Separation of Electron’s Intrinsic Properties Revealed
Disentanglement reveals exotic magnetic properties in a ytterbium-based compound.
The Science
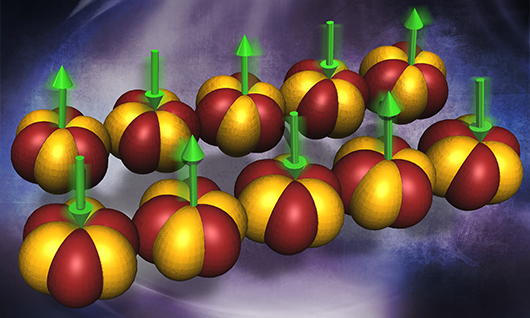
Today’s computers rely on the magnetic behavior of key compounds. New materials with unusual magnetic properties are vital for faster computers for science, security, and other technologies. Neutron scattering characterization revealed an unexpected magnetic behavior in a three-dimensional magnetic material made of ytterbium, platinum, and lead (Yb2Pt2Pb). The behavior is similar to that in a one-dimensional chain of magnetic moments. Scientists showed that this unusual phenomenon, seen for the first time in a three-dimensional material, arises due to hopping of electrons. The electrons hop between the orbitals of neighboring atoms (orbital exchange). The electrons create the one-dimensional magnetism.
The Impact
The discovery provides yet another magnetic property that could be harnessed. These properties could aid in new approaches to high-performance computing and energy-efficient technologies.
Summary
An electron in an atom has three intrinsic properties: charge, spin and orbital angular momentum; the latter characterizes how the electron orbits around the nucleus. This can be understood by comparing electrons to the planets in our planetary system. Planets have their own angular momentum based on their fixed orbits around the sun. When electrons in a solid are constrained, like the planets, to only move along one direction, their properties, such as charge and spin can separate and behave as independent entities. The properties can be exploited for various applications. For example, the electron’s charge has been used in all semiconductor-based electronics, and its spin has been controlled and manipulated for spintronics applications. What has not been demonstrated until now is the separation of charge and orbital degrees of freedom, and this study completes the triad of what is called fractionation of the three properties of electrons. A team led by researchers at Stony Brook University and Brookhaven National Laboratory discovered unexpected magnetic behavior in Yb2Pt2Pb from neutron scattering measurements on a single crystal at temperatures close to absolute zero (nearly -460 °F). The scientists expected to find a spectrum (intensity of scattered neutrons as a function of energy and momentum resulting from their interactions with the material), showing sharply defined peaks or excitations. The peaks or excitations would correspond to the wave-like swinging motion of the large magnetic moments that result from the collective orbital angular momentum of several electrons per atom. The researchers were puzzled to discover a continuum of excitations with the precise form theoretically predicted for a one-dimensional chain of magnetic moments corresponding to the spin of a single electron. An explanation for this conundrum came from considering what happens when electrons hop between atomic orbitals in neighboring ytterbium (Yb) ions. Based on the quantum mechanical selection rules, Yb atoms can only make low-energy electron exchanges with neighbors along one direction. Because of a strong coupling between the electronic spins and the atomic orbitals, the electron exchange causes the large magnetic moments on neighboring sites to flip their orientations. The discovery of the orbital exchange in magnetic materials offers yet another route to harness magnetism for applications in information and energy technologies.
Contact
Igor Zaliznyak
Brookhaven National Laboratory
zaliznyak@bnl.gov, 631-344-3761
John Tranquada
Brookhaven National Laboratory
jtran@bnl.gov, 631-344-7547
Funding
- U.S. Department of Energy (DOE) Office of Science, Basic Energy Sciences supported work at Brookhaven National Laboratory and at the Spallation Neutron Source, a DOE Office of Science user facility
- National Science Foundation supported work at Stony Brook University and facilities at the National Institute of Standards and Technology Center for Neutron Research
- Netherlands Organization for Scientific Research and the Foundation for Fundamental Research on Matter supported theoretical work in the Netherlands
Publications
L.S. Wu, W.J. Gannon, I.A. Zaliznyak, A.M. Tsvelik, M. Brockmann, J.S. Caux, M.S. Kim, Y. Qiu, J.R.D. Copley, G. Ehlers, A. Podlesnyak, and M.C. Aronson, “Orbital-exchange and fractional quantum number excitations in an f-electron metal, Yb2Pt2Pb.” Science 352, 1206 (2016). [DOI: 10.1126/science.aaf0981]
Related Links
Brookhaven National Laboratory news release: Scientists Find Surprising Magnetic Excitations in a Metallic Compound
Oak Ridge National Laboratory news release: Neutrons Reveal Unexpected Magnetism in Rare-Earth Alloy
Highlight Categories
Performer: University , DOE Laboratory , SC User Facilities , SNS
Additional: Collaborations , Non-DOE Interagency Collaboration