Neutrons Reveal New Electrocatalyst Family for Producing Hydrogen Fuel
New catalyst material provides potential for cheaper renewable hydrogen production.
The Science
Researchers observed very high activity for hydrogen fuel production from water in a family of catalysts that contain cobalt and other metals whose structure was shown by neutron diffraction experiments to differ from that of other known catalysts.
The Impact
Cobalt molybdenum nitride compounds developed in this study efficiently catalyze the conversion of electrical energy to chemical energy, which can be stored with about a hundred-fold improvement in energy density. Although the present cobalt molybdenum nitride catalysts are more than 10% less efficient during the energy conversion process than the best known catalysts, further optimization of this novel catalyst family may produce catalytic performance closely matching or even exceeding that of expensive noble metal systems currently in use.
Summary
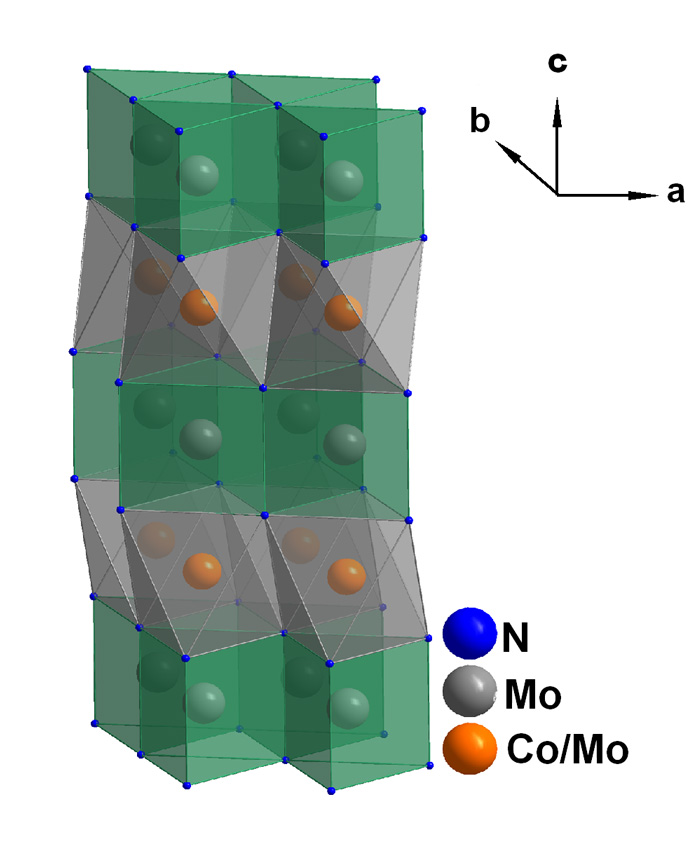
Most renewable energy is produced in the form of electricity, which is expensive to store and transport in large quantities. Producing hydrogen from water using the hydrogen evolution reaction (HER) provides an alternative renewable fuel that is more easily stored and transported than electricity generated through competing photovoltaic (PV) technologies. Due to the energy barriers of the reactions, catalysts are essential to increase the overall hydrogen production efficiency, which is lower than that of direct generation of electricity. Unfortunately, the best HER catalysts incorporate noble metals such as the rare and expensive platinum, which limits the viability of cheap, renewable hydrogen production. Researchers recently discovered a family of cobalt molybdenum nitrides with very high catalytic activity for hydrogen fuel production from water but whose chemical structure differs from known catalysts for this reaction. The atomic structures of these compounds were determined by neutron diffraction on small (~100 mg) powder samples using the Oak Ridge National Laboratory Spallation Neutron Source’s Nanoscale-Ordered Materials Diffractometer (NOMAD) instrument. The strong sensitivity of neutron diffraction to light atoms made it possible to determine the unique location of the nitrogen atoms in the catalyst material and confirm that the superior catalytic activity of the compound Co0.6Mo1.4N2, compared to binary MoN, was due to the octahedral Mo environment.
Contact
Peter Khalifah
Brookhaven National Laboratory and Stony Brook University (Joint Appointment)
631-344-7689
kpete@bnl.gov
Funding
Work performed at the Oak Ridge National Laboratory Spallation Neutron Source’s Nanoscale-Ordered Materials Diffractometer (NOMAD) instrument was supported by Scientific User Facilities Division, Office of Basic Energy Sciences, US Department of Energy. Work carried out at Brookhaven National Laboratory’s Chemistry Department and Center for Functional Nanomaterials was primarily supported by Brookhaven Laboratory Directed Research and Development funds.
Publications
B. Cao, G. M. Veith, J. C. Neuefeind, R. R. Adzic, and P. G. Khalifah. “Mixed Close-Packed Cobalt Molybdenum Nitrides as Non-noble Metal Electrocatalysts for the Hydrogen Evolution Reaction.” Journal of American Chemical Society 135 (2013): 19186–19192. [DOI: 10.1021/ja4081056]
Highlight Categories
Performer: DOE Laboratory , SC User Facilities , BES User Facilities , SNS , CFN