Nanoparticles: To Be or Not to Be Crystalline?
Discovery of coexisting ordered and disordered catalytic nanoparticles.
The Science
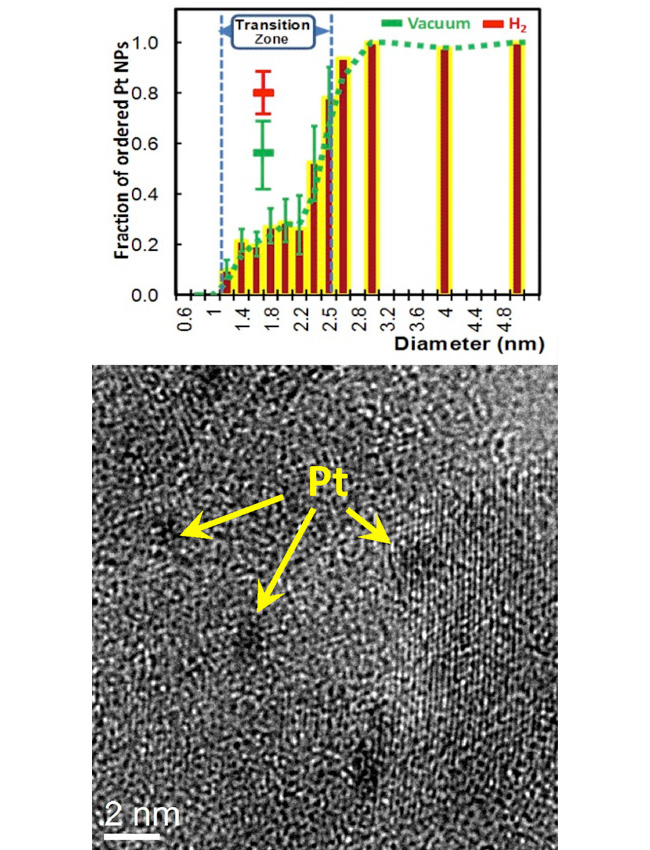
By changing the size of small metal nanoparticles, their properties (e.g., shape, structure, and chemistry) can be tuned and perhaps controlled unlike individual atoms or bulk material. Researchers discovered that there is no one size where particles go from non-crystalline (disordered) to crystalline (ordered) phases, but instead found a range of sizes where both phases coexist statistically, depending on the support and gas surrounding the particles.
The Impact
By controlling size and shape, the properties of metal nanoparticles can be tuned to catalyze chemical reactions faster and/or potentially cheaper for a wide range of fields including those related to energy production as well as biomedical, chemical, electronic, and optical technologies. One must now design statistical collections of nanoparticles to optimize the reaction and performance – depending on size, what supports the nanoparticles, and what chemicals and gases are involved.
Summary
The synthesis and unusual size-dependent properties of nanostructures between 1-10 nm, including metal nanoparticles (NPs), are the subject of intense research due to their exceptional promise in advancing catalysis for fuel and chemical manufacturing as well as drug synthesis and magnetic devices. Due to its role in energy production and, hence, worldwide economy, catalysis depends on NPs structure and the substrate and gaseous environment. Though descriptions of bulk and atomic properties of matter are well established, critical questions remain about the behavior and kinetics of nanoparticles in mesoscopic regimes. To address these questions, the structure, chemical state, and phase transformations of platinum (Pt) nanoparticles were characterized in real time and in their operational environments. The non-crystalline-to-crystalline transition of supported Pt NPs in the sub-nanometer to nanometer size range was shown to be statistical in nature, and strongly affected by particle size, support, and adsorbates. Unlike in bulk conditions, a non-crystalline phase exists that is stable. Observations of over 3000 particles by high-resolution transmission electron microscopy show a non-crystalline-to-crystalline transition zone that is non-abrupt – there is a size regime where disordered and ordered NPs coexist. The NP size at which this transition occurs is strongly dependent on both the adsorbate and the support.
Contact
Duane Johnson
The Ames Laboratory/US Department of Energy
ddj@ameslab.gov
Funding
This research was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geochemical and Biochemical Sciences Division (DEFG02- 03ER15476). The Environmental TEM measurements were carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory (DE-AC02-98CH10886). Statistical studies of nanoparticle visibility as function of support thickness was performed at the National Center of Electron Microscopy, Lawrence Berkeley National Laboratory (DOE-BES: DE-AC02-05CH11231). The theory and simulations were performed at the Ames Laboratory, which is operated for the DOE by Iowa State University (DE-AC02-07CH11358). The High-Resolution TEM measurements were conducted at the Nanoscale Fabrication and Characterization Facility (NFCF) at the University of Pittsburgh.
Publications
Long Li, Lin-Lin Wang, Duane D. Johnson, Zhongfan Zhang, Sergio I. Sanchez, Joo H. Kang, Ralph G. Nuzzo, Qi Wang, Anatoly I. Frenkel, Jie Li James Ciston, Eric A. Stach, and Judith C. Yang, “ Noncrystalline-to-Crystalline Transformations in Pt Nanoparticles,” Journal of American Chemical Society 135, 13062-13072 (2013). [DOI: http://dx.doi.org/10.1021/ja405497p]
Related Links
Chosen as Editor’s Suggestion: Nanoparticle Transformers, by Marc S. Lavine, Science 9 August 2013: 593. [DOI: http://dx.doi.org/10.1126/science.341.6146.593-c]
Highlight Categories
Performer: DOE Laboratory , SC User Facilities , BES User Facilities , CFN , NCEM