Discovery of New Materials to Capture Methane
Predicted materials could economically produce high-purity methane from natural gas systems and separate methane from coal mine ventilation systems.
The Science
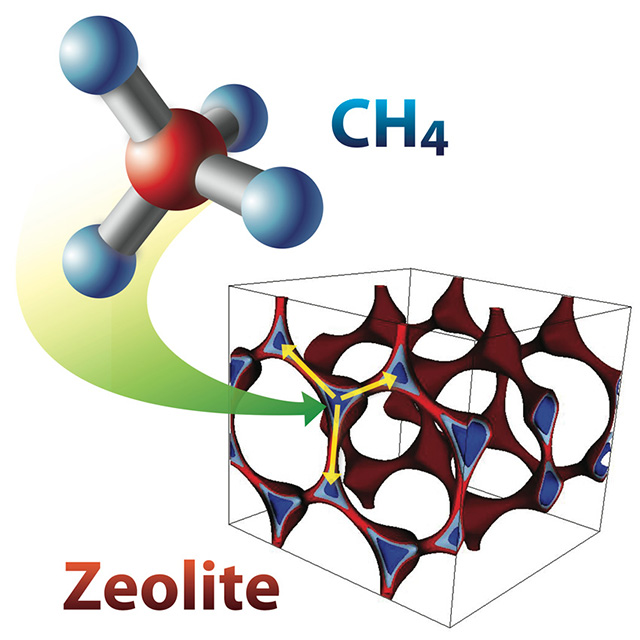
Computer simulations reveal new zeolites (porous crystalline adsorbents used by oil industries) with sufficient methane (CH4) adsorption capacity and better CH4/carbon dioxide and CH4/nitrogen selectivity to be technologically promising for methane capture from dilute and medium-concentration sources.
The Impact
Low cost separation of high-purity methane from low quality natural gas sources has long been sought, and could provide a new commercial source of methane to use as fuel or chemical feedstock. Capturing methane emitted from a variety of low concentration sources will help reduce its powerful greenhouse gas contribution to global climate change and improve coal mine safety by decreasing methane concentrations.
Summary
Methane, a common gas emitted from natural gas systems, landfills, coal mining, waste water treatment and hydrates in the ocean, is both a great energy source and a greenhouse gas with a global warming potential over 20 times that of carbon dioxide (CO2). Methane often coexists with CO2 and nitrogen gas (N2). Effective techniques are badly needed for the economical separation of methane from these gases to use methane as a fuel and to reduce its environmental impact. However, this has proved challenging because methane molecules are nonpolar, that is, an overall charge-neutral system with a highly symmetric structure that makes it interact weakly with most materials systems. To address this knowledge gap, a team of scientists from the Center for Gas Separations Relevant to Clean Energy Technologies EFRC at Lawrence Berkeley National Laboratory collaborated with scientists at Lawrence Livermore National Laboratories to develop novel computational approaches to screen over 87,000 possible zeolites (porous crystalline adsorbents used by oil industries). This study discovered a few zeolite structures that are technologically promising – they have both sufficient methane sorption capacity and excellent selectivity for the separation of methane from mixtures with CO2 and/or N2. Realization of these new materials could enable natural gas industries to economically produce high purity methane from natural gas systems and could be used to separate methane from a variety of low concentration sources to reduce methane’s environmental impact and enhance safety in closed environments in which high methane concentrations could result in explosions.
Contact
Berend Smit
Director, Center for Gas Separations Relevant to Clean Energy Technologies (CGS) EFRC
Berend-Smit@Berkeley.edu
Funding
DOE Office of Science, Basic Energy Sciences, Energy Frontier Research Centers (EFRC) Program (methodology, computer codes, screening solids, and potentials, B. S.)
DOE Office of Science, National Energy Research Scientific Computing Center (computational resources)
DOE Fossil Energy (data for CO2/CH4 adsorption J. K.)
DOE ARPA-E (screening of liquids at Lawrence Livermore National Laboratory and data for CO2/N2 adsorption J.K.)
Deutsche Forschungsgemeinschaft (L.-C.L. fellowship)
Publications
Jihan Kim, Amitesh Maiti, Li-Chiang Lin, Joshuah K. Stolaroff, Berend Smit & Roger D. Aines, “New materials for methane capture from dilute and medium-concentration sources”, Nature Communications, 4, 1694 (2013) 16th April 2013, [DOI: 10.1038/ncomms2697].
Related Links
Center for Gas Separations Relevant to Clean Energy Technologies (CGS) EFRC
Lawrence Berkeley National Laboratory News
Lawrence Livermore National Laboratory News
Highlight Categories
Performer: University , DOE Laboratory , SC User Facilities , ASCR User Facilities , NERSC
Additional: Collaborations , ARPA-E , FE