Catching Lithium Ions in Action in a Battery Electrode
New microscopy with nanometer-sized resolution may bring revolutionary new understanding to energy storage technologies.
The Science
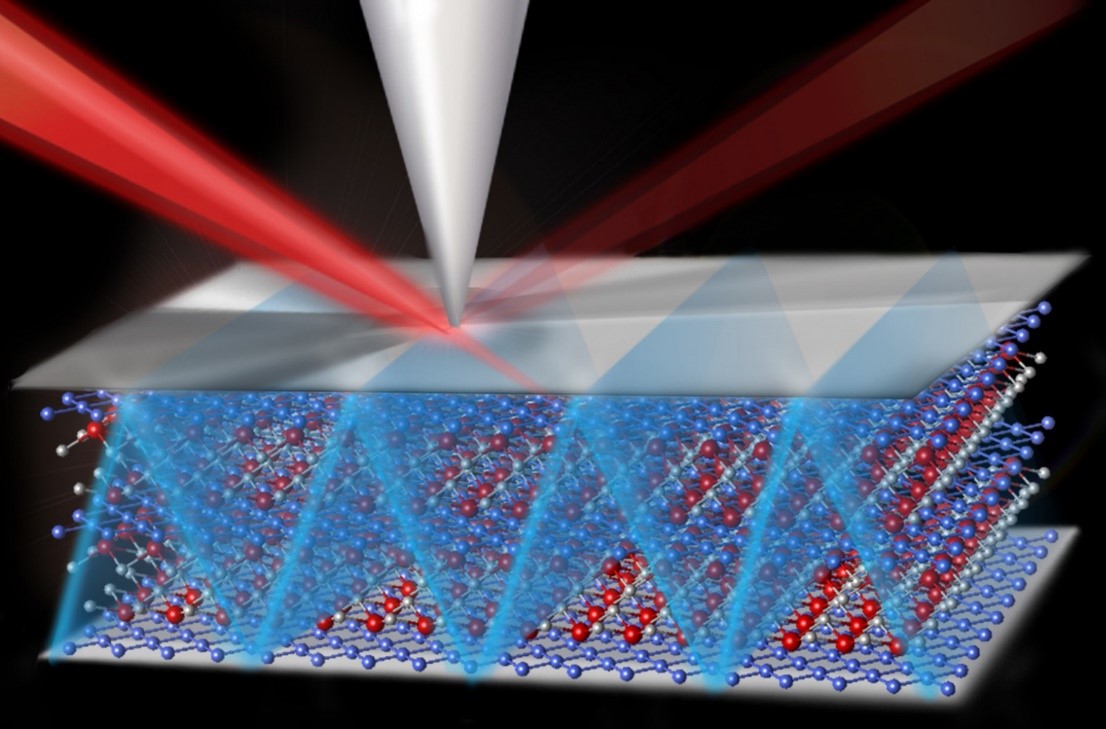
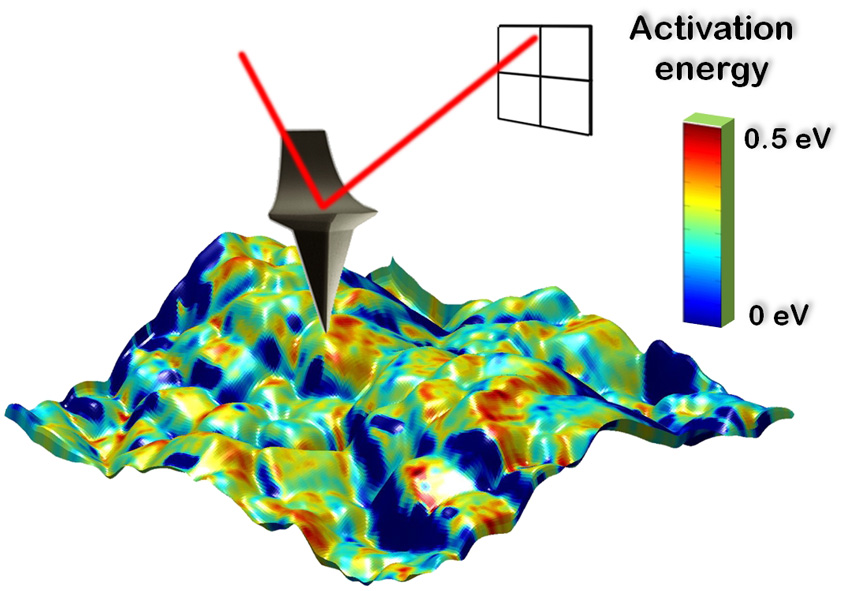
We can now measure and understand, for the first time, Li-ion diffusion and electrochemical reactions on a nano-metric scale. New electrochemical strain microscopy (ESM) has been developed and used to measure Lithium-ion activation energies with nano-meter resolution, revealing important new findings.
The Impact
The performance of current Lithium-ion batteries has been well below the theoretical limits and expectations of practical applications, because we could not measure and understand the fundamentals of charge storage on a nano-scale. This new capability promises to bring a significant new understanding to these limiting properties.
Summary
A new scanning probe technique has led to the first measurements of the activation energy for Li-ion transport with nanometer resolution in the battery electrode material LiCoO2. Understanding ionic transport at the level of individual grains and grain facets is of great importance in improving future energy storage (battery) energy and conversion (fuel cell) devices. Until now, activation energies for ionic transport have been actively explored by electrochemical techniques on the macroscopic and device level, allowing only average values for the activation energy to be determined. In this work, temperature-dependent electrochemical strain microscopy (ESM) is used to measure the activation energy of Li-ion transport in LiCoO2 thin films on the nanometer scale, bridging the lengths scales of atomistic calculations and traditional macroscopic experiments. By understanding the local picture of Li-ion transport in electrode materials and its correlation with the microstructure, a better understanding of ionic flow through a battery can be developed, as is required for future improvements in battery technologies.
Contact
Nina Balke
Associate Research
Staff Member Oak Ridge National Laboratory
P. O. Box 2008
Oak Ridge, TN 37831-6487
balken@ornl.gov
Funding
Basic Research: Office of Science Basic Energy Sciences (BES) program, Materials Sciences and Engineering Division (MSE) and Scientific User Facilities Division (SUF).
Applied Research: Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Program (provided samples for characterization)
Publications
N. Balke, S. Kalnaus, C. Daniel, N. J. Dudney, S. Jesse, S. V. Kalinin, Local Detection of Activation Energy for Ionic Transport in Lithium Cobalt Oxide, Nano Lett. 12, 3399-3403 (2012).
Highlight Categories
Performer: DOE Laboratory , SC User Facilities , BES User Facilities , CNMS , ShaRE
Additional: Collaborations , EERE