Underground Storage of Carbon Dioxide—as a Solid
Nanoscale features in rocks enable more carbon dioxide to be trapped as a solid carbonate material underground.
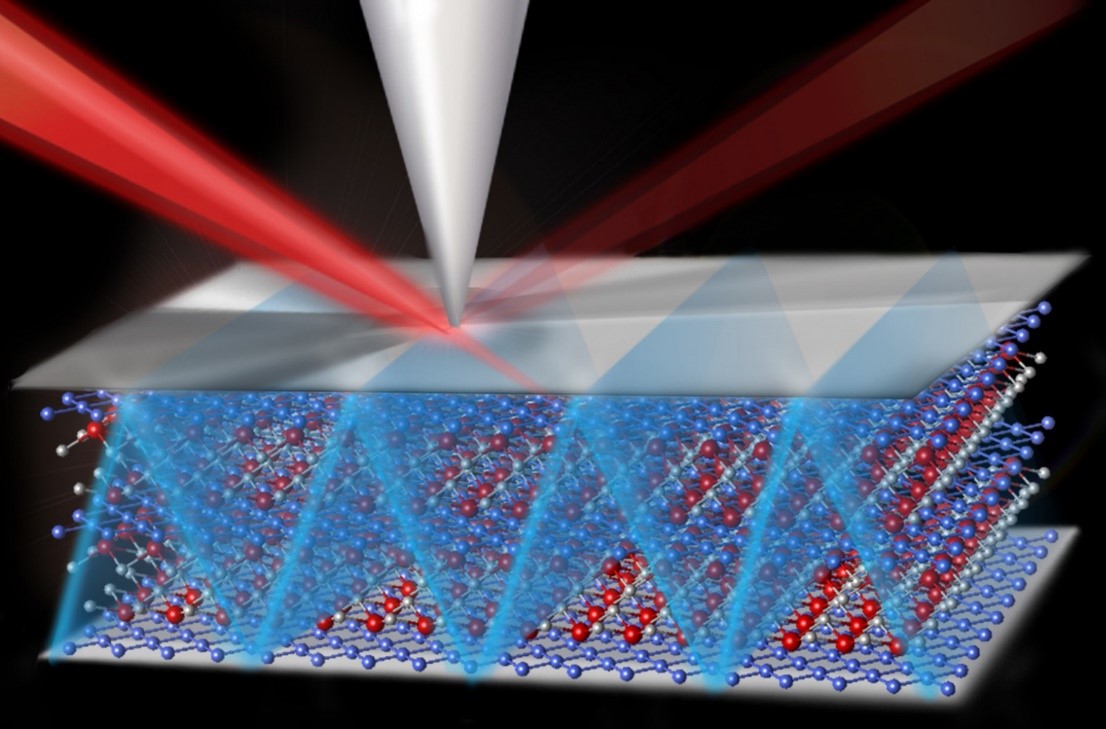
The Science
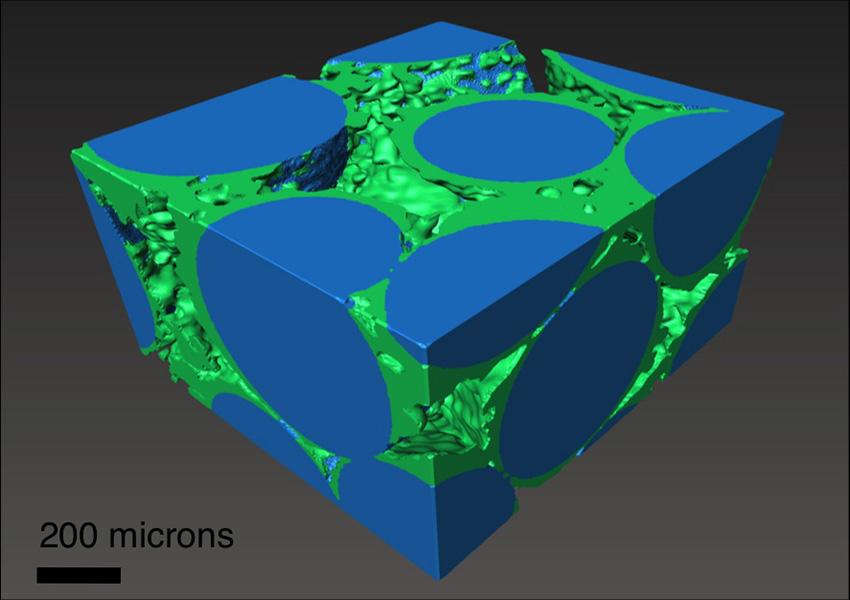
Nanoscale features of natural rock surfaces accelerate the nucleation and growth of carbonate minerals, the thermodynamically favored form of carbon dioxide (CO2) in geologic formations. This research used advanced experiments and computational modeling to probe these nanoscale features and discover how they control the growth and distribution of solid carbonates.
The Impact
This research enables more realistic predictions of how carbonate growth is localized in porous rocks and how it influences the flow of CO2 through the rock and the reactivity of the rocks with CO2. These results can be readily used to improve the accuracy of models that predict what happens to CO2 stored in underground geologic formations on scales of tens of square miles.
Summary
Advanced experiments and computations have shown that underground carbonate mineral nucleation and growth is strongly dependent on nanoscale features such as the pore structure and surface topography of permeable rocks and the interfacial energies between rock surfaces and solid carbonates. This research at the Lawrence Berkeley National Laboratory’s Center for Nanoscale Control of Geologic CO2, Washington University in St. Louis, and Oregon State University provides the quantitative parameters necessary to develop advanced models that describe how nucleation and growth of carbonate occur in porous media that contain multiple minerals with different surface properties and micro- to nanoscale pores. In carbon capture and storage, CO2 is captured from power plant exhaust and other sources and injected underground into porous rock formations where it mixes with ambient salt water and may remain for 1000’s of years. Although it is expected that CO2 can be transformed to carbonate minerals, it is unknown how fast this will occur and how the addition of new carbonate mineral in the rock formations will affect the short and long-term behavior of the system. This research will enable more realistic modeling of mineral formation from the injected CO2 and thus increase the pace of deployment of this critical energy technology.
Contact
Don DePaolo
Center for Nanoscale Control of Geologic CO2 (NCGC) EFRC
Lawrence Berkeley National Laboratory
depaolo@eps.berkeley.edu
Funding
Basic Research: DOE Office of Science, Office of Basic Energy Sciences, Energy Frontier Research Centers (EFRC) Program and the Office of Biological and Environmental Research. The research utilized the following Office of Science user facilities: Advanced Light Source (ALS), Advanced Photon Source (APS), Molecular Foundry, and National Energy Research Scientific Computing Center (NERSC).
Publications
R. Armstrong, J. Ajo-Franklin, “Investigating biomineralization using synchrotron based X-ray computed microtomography.” Geophysical Research Letters 38, L08406 (2011). [DOI: 10.1029/2011GL046916]
A. Fernandez-Martinez, Y. Hu, B. Lee, Y.-S. Jun, G.A. Waychunas, “In situ determination of interfacial energies between heterogeneously nucleated CaCO3 and quartz substrates: Thermodynamics of CO2 mineral trapping.” Environmental Science and Technology 47, 102-109 (2013). [DOI: 10.1021/es3014826]
L.O. Hedges, S. Whitelam, “Patterning a surface so as to speed nucleation from solution.” Soft Matter 8, 8624-8635 (2012). [DOI: 10.1039/C2SM26038G]
Related Links
Center for Nanoscale Control of Geologic CO2 (NCGC) EFRC
http://esd.lbl.gov/research/facilities/ncgc/
Highlight Categories
Program: ASCR , BES , EFRCs , BER , BSSD
Performer: University , DOE Laboratory , SC User Facilities , ASCR User Facilities , NERSC , BES User Facilities , ALS , APS , Foundry
Additional: Collaborations , FE