Hydrogen Production Forwards and Backwards
A single reversible catalyst enables energy to be both stored and released on demand.
The Science
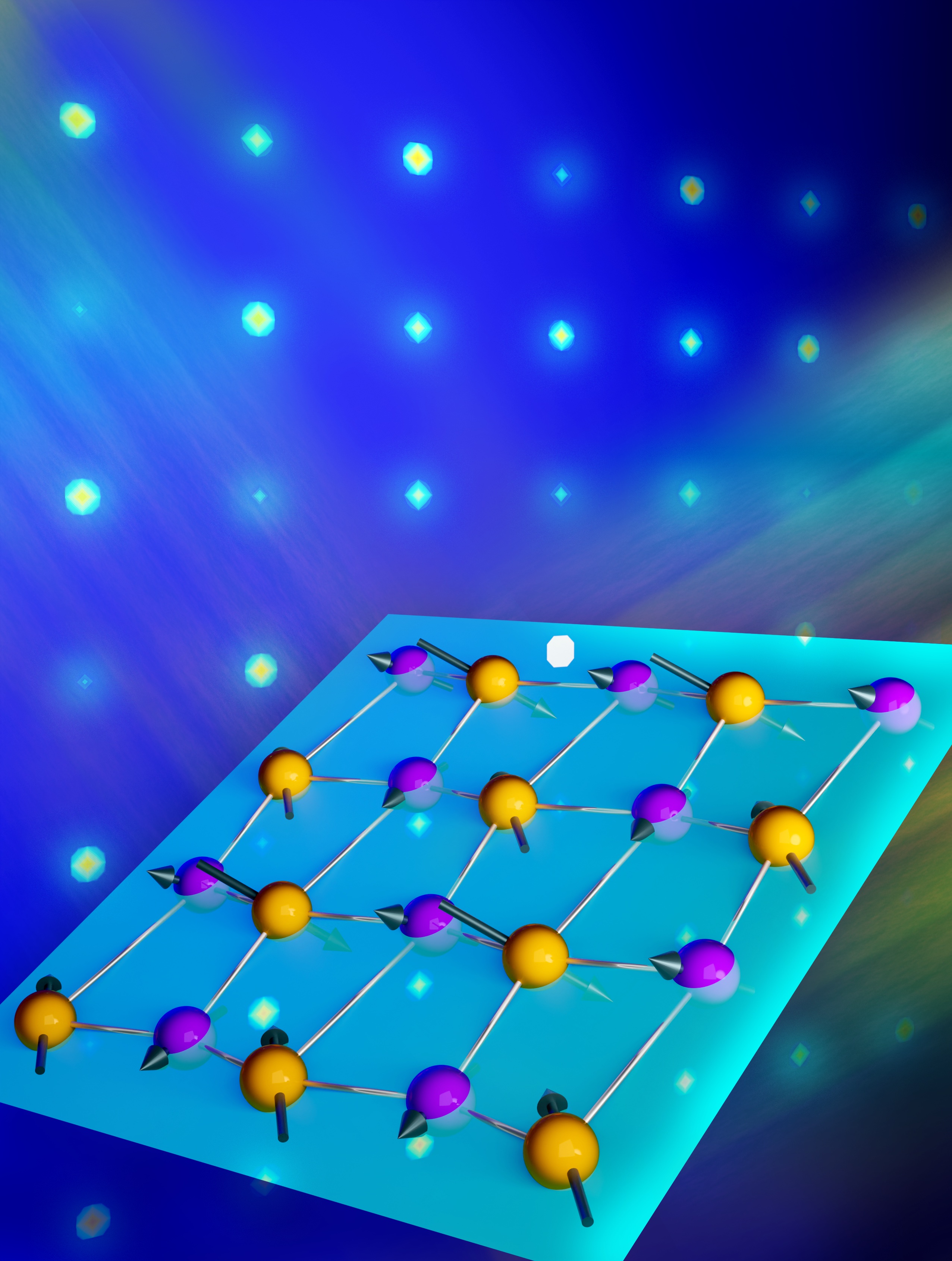
Using nature as a guide, researchers developed an inexpensive catalyst that can produce hydrogen from electricity and can later convert the stored hydrogen back into electricity.
The Impact
These results present a new path for inexpensively converting electrical energy generated by wind or solar farms to a fuel (hydrogen) that can be stored and converted back to electricity when needed.
Summary
Alternative energy sources like wind and solar produce intermittent power that must be stored for later use when the wind stops blowing or the sun sets. One storage mechanism is to transform electrical energy from solar and wind into chemical energy like hydrogen, but this can be complex and costly. To produce hydrogen efficiently, Center for Molecular Electrocatalysis (CME) researchers are designing a hydrogen splitting catalyst based on the hydrogenase enzyme found in nature. Hydrogenase cleaves two linked hydrogen atoms (dihydrogen) into electrical energy, and it can reverse the reaction to form dihydrogen. This enzyme also has features CME researchers want – a single catalyst that works at ambient temperature and pressure, and an active site that uses abundant metals such as iron or nickel. To build the synthetic catalyst, molecular strands called ligands were attached to a nickel active site. Using theory and experiments, researchers varied size, shape, and electronic properties of the ligands to “tune” catalytic activity. One nickel complex displayed reversible dihydrogen production and cleavage with high efficiency – the first example of a molecular complex to catalyze these reversible reactions. While the catalyst is slow, it wastes little energy and marks an important first step towards improving capabilities for energy storage.
Contact
Jenny Yang
California Institute of Technology
jyy@caltech.edu
R. Morris Bullock
Director of the Center for Molecular Electrocatalysis EFRC
morris.bullock@pnnl.gov
Funding
DOE Office of Science, Basic Energy Sciences, Energy Frontier Research Centers (EFRC) Program
Publications
Smith, Stuart E; Yang, Jenny Y; DuBois, Daniel L; and Bullock, R. Morris “Reversible Electrocatalytic Production and Oxidation of Hydrogen at Low Overpotentials by a Functional Hydrogenase Mimic” Angew. Chem. Int. Ed., 51, 3152-3155 (2012). [DOI: 10.1002/anie.201108461]
Related Links
DOE Office of Science, Stories of Discovery & Innovation
Center for Molecular Electrocatalysis EFRC
Highlight Categories
Performer: University , DOE Laboratory