Peering into the Flame
New insights from synchrotron-based studies are helping to assess the potential of new biofuels.
The Science
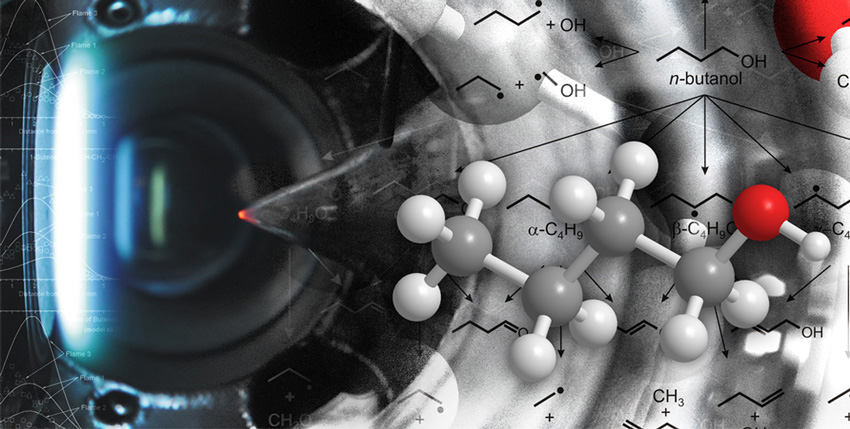
The fidelity of a combustion chemistry computational model for butanol, a potential biofuel, was determined by experimentally quantifying seldom observed reaction intermediates predicted by theoretical calculations.
The Impact
The ability to accurately predict combustion chemistry with computational models can lead to faster, more cost-effective assessment of proposed new fuels.
Summary
Evaluation of new fuels in engines is hampered by both expense and need for large quantities of the test fuel — a problem given the limited availability for many potential biofuels. Predictive computer modeling based on quantum mechanics and chemistry promises to reduce the number of experiments needed for evaluation. To validate the fidelity of combustion chemistry models, researchers at the CEFRC conducted rigorous experimental tests of the computer predictions for combustion of butanol, an alternative fuel nearing commercialization. Using the Advanced Light Source, a synchrotron that generates bright beams of x-rays capable of revealing atomic and electronic structure of matter, the scientists were able to quantify the chemical species in butanol flames, including species not observable using ordinary techniques. While showing that the model predictions resemble the experimental data and are generally accurate, the research identified areas for improvement. Overall, the results suggest the computer modeling approach currently being developed will accelerate the evaluation of proposed fuels while reducing cost and fuel use.
Contact
N. Hansen
Sandia National Laboratories
nhansen@sandia.gov
W. H. Green
MIT
whgreen@mit.edu
Chung K. Law
Director of the Combustion Energy Frontier Research Center (CEFRC)
cklaw@princeton.edu
Funding
DOE Office of Science, Office of Basic Energy Sciences, Energy Frontier Research Centers (EFRC) Program; Advanced Light Source (ALS)
Publications
Hansen, N.; Harper, M.R.; and Green, W.H. “High-temperature oxidation chemistry of n-butanol – experiments in low-pressure premixed flames and detailed kinetic modeling” Phys. Chem. Chem. Phys., 13, 20262–20274 (2011). [DOI: 10.1039/c1cp21663e]
Related Links
Combustion Energy Frontier Research Center
Highlight Categories
Performer: University , DOE Laboratory , SC User Facilities , BES User Facilities , ALS