A Better Way to Make Biodiesel Fuel
New, non-toxic catalysts have been commercialized and may lower production cost for biodiesel.
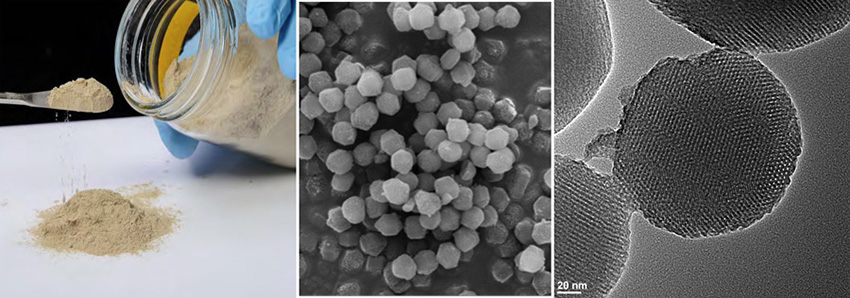
The Science
Co-locating catalysts and substrates in confined spaces was found to greatly increase reaction rates and resulted in the discovery of a new series of catalysts for biodiesel production. Application of this knowledge led to the creation of a new catalyst that converts vegetable and algal oils to biodiesel and proved to be active all the way to the pilot-plant scale.
The Impact
This non-toxic, drop-in replacement catalyst works on a broad spectrum of feedstocks, even with impurities, and could lower the cost of producing biodiesel. Today, the catalyst is a commercial product and is used to produce high quality biodiesel.
Summary
Many of the key technical and economic challenges of using non-food-based oils for biodiesel production can now be overcome by basic research on catalyst synthesis. Scientists at the Ames Laboratory discovered cooperative solid catalyst technologies that are highly efficient at converting vegetable oils, animal fats, and waste oils into biodiesel. Fats and oils are triglycerides, meaning they contain a glycerol molecule (a type of alcohol) linked to three fatty acid chains. For biodiesel production, the fatty acids are separated from the glycerol and then bonded to a different alcohol in a process called transesterification. Current biodiesel conversion catalysts require high temperature and high pressure and are only effective for pure feedstocks or a single type of fat molecule, making them less efficient for commercial strategies. Research on mixed metal oxides in confined spaces provided the necessary platform to custom design a new class of catalysts that can convert mixed feedstocks and work under milder conditions. After a successful technology transfer to Catilin, Inc. (now a subsidiary of Albemarle Corporation), followed by scale-up synthesis and demonstration at a pilot-plant, the heterogeneous catalyst is fully commercialized. Marketed as GoBio T300, this novel drop-in solid catalyst is nontoxic and is a direct replacement for conventional catalysts used in biodiesel production. Because GoBio T300 catalysts operate at industry standard pressures and temperatures, and can be removed by filtration, current producers can easily retrofit their biodiesel plants in a matter of days at very low cost. This catalyst lowers the cost of producing biodiesel relative to more traditional processes as well as produces a high quality, sellable co-product of glycerin used in pharmaceuticals.
Contact
Cynthia Jenks
Ames Laboratory
Chemical and Biological Sciences Division Director
cjenks@ameslab.gov
Funding
Basic Research: DOE Office of Science, Office of Basic Energy Sciences and the US Department of Agriculture – DOE R&D Biomass Initiative
Follow-up Applied R&D: DOE Office of Energy Efficiency and Renewable Energy, Biomass Program
Publications
T.M. Hsin et al. “Calcium containing silicate mixed oxide-based heterogeneous catalysts for biodiesel production” Topics in Catalysis, 2010, 53, 746-754. [DOI: 10.1007/s11244-010-9462-3]
Related Links
http://www.biodieselmagazine.com/articles/3536/a-solid-catalyst-unlike-the-rest
Highlight Categories
Performer: DOE Laboratory
Additional: Technology Impact , Collaborations , EERE , Non-DOE Interagency Collaboration