Terminating Toxic Metals in the Environment
Understanding ceramic chemistry leads the way to a new remediation technology
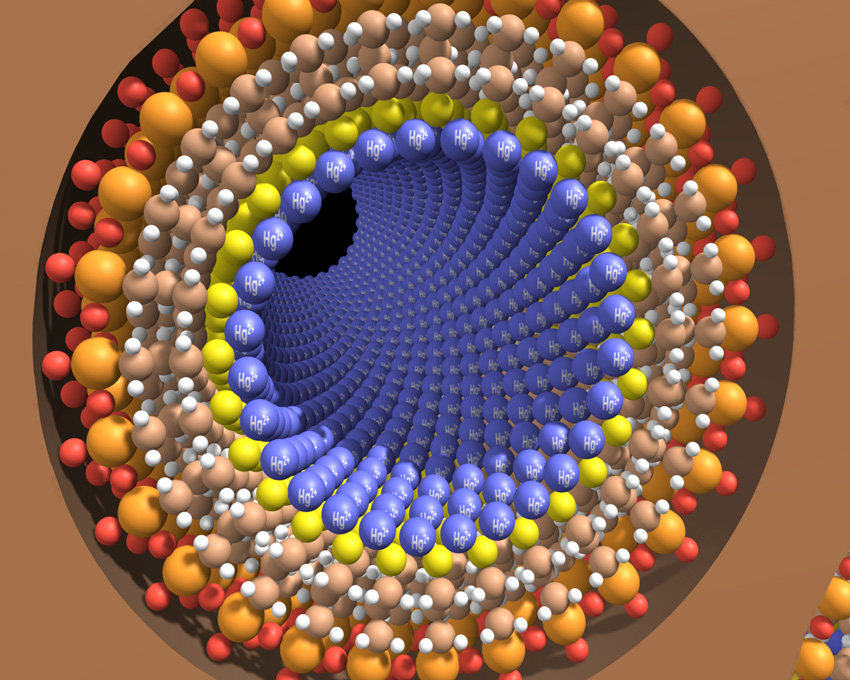
The Science
Meso-porous silica materials were designed and synthesized with ligands to attract and hold contaminants such as mercury and other heavy metals.
The Impact
This technology allows remediation of complex environmental contamination issues, including the removal of mercury, without creating hazardous waste or by-products.
Summary
Basic research at Pacific Northwest National Laboratory developed a cross-disciplinary technology that coats mesoporous silica material with synthesized ligands, or binding molecules, which can serve many purposes including attracting and holding contaminants, such as mercury. This technology, called SAMMS short for Self-assembled Monolayers on Mesoporous Supports, self-assembles a single layer of ligand onto the mesporous ceramic or silica supports. Because the ceramic or silica is so porous, the high surface area creates a high density (≈1000 m2/g) of contamination binding sites. A small amount – about a tablespoon – has the equivalent surface area of a football field, with binding molecules covering the available surface. These properties add up to a product that is about 500 times faster and much less expensive than previous mercury remediation methods. Due to its structure and chemistry, SAMMS can be modified to meet broader needs. The technology is used to treat groundwater, contaminated mining impoundments, industrial process streams, and contaminated oil. SAMMS is also being used to sequester a variety of contaminants, including heavy metals like mercury and lead, anions like arsenate and selenite, and radioactive materials like radiocesium and radioiodide. Commercialized through Steward Advanced Materials, SAMMS recognitions include an R&D 100 Award and Popular Science magazine‘s Grand Award winner for Green Technologies in their Best of What’s New Awards in 2009.
Contact
J. Liu, PNNL
jun.liu@pnnl.gov
Doug Ray, PNNL
doug.ray@pnnl.gov
Funding
Basic Research: Office of Science Basic Energy Sciences (BES) program and Biological and Environmental Research (BER) program; PNNL's Laboratory Directed Research and Development (LDRD) program supported initial research.
Applied R&D: DOE Office of Environmental Management (EM).
Publications
Chen, XB, et al. "Mercury separation and immobilization using self-assembled monolayers on mesoporous supports (SAMMS)". Separation Science and Technology 34: 1121-1132 (1999). [DOI: 10.1080/01496399908951084].
Feng X, GE Fryxell, LQ Wang, AY Kim, and J Liu. "Organic Monolayers on Ordered Mesoporous Supports." Science 276, 923-926 (1997).
Fryxell GE, et al. “Design and Synthesis of Self Assembled Monolayers on Mesoporous Supports (SAMMS): The Importance of Ligand Posture in Functional Nanomaterials.” Journal of Materials Chemistry 17, 2863 - 2874 (2007).
Related Links
http://www.pnl.gov/science/highlights/highlight.asp?id=1030
http://www.pnl.gov/nano/research/pdf/samms_flier_12-03-2010.pdf
http://samms.pnnl.gov/index.stm
SAMMS® Adsorbents by Steward Advanced Materials
Highlight Categories
Program: BES , MSE , BER , CESD
Performer: DOE Laboratory , SC User Facilities , BES User Facilities , APS , NSLS
Additional: Technology Impact , Collaborations , EM