No Honor Among Copper Thieves
Microbial Cheaters and their Impact on Greenhouse Gas Emissions
The Science
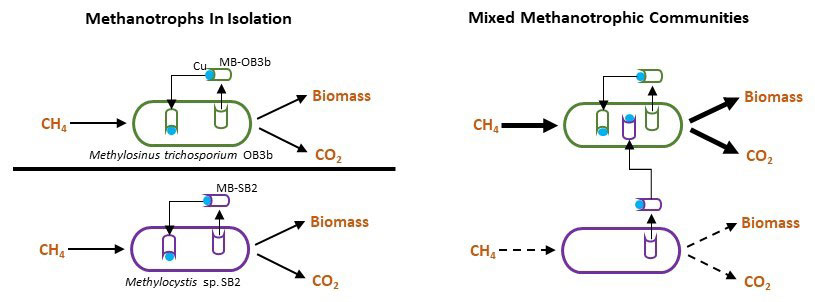
As John Donne wrote “no man is an island,” and similarly no microbe is truly alone. Microbes, like people, interact with each other in many different ways. Sometimes they cooperate, sometimes they compete, and sometimes they “steal” from one another. A remarkable example is how methanotrophs or “methane-eaters” interact. Methanotrophs need copper in order to consume methane. To that end, methanotrophs have evolved strategies to collect copper by producing a copper-binding compound named methanobactin. But some methanotrophs actively take up methanobactins produced by other methanotrophs to fulfill their own copper needs. Such “theft” makes them more competitive than others and controls how the overall community consumes methane.
The Impact
Methanotrophs play an important role in eliminating methane, a potent greenhouse gas. To oxidize methane, they need copper. The mineral is an essential part of these microorganisms’ cellular machinery. By understanding how methanotrophs collect copper, scientists can manipulate this activity and increase how much methane the microbes consume.
Summary
Copper is an important component for the metabolism of aerobic methanotrophs, as it controls the expression and activity of alternative forms of methane monooxygenase (MMO). To collect copper, some methanotrophs secrete a peptide—methanobactin (MB)—that has high affinity for copper. After binding copper, MB is taken back up via a TonB-dependent transporter (TBDT), a protein in the microbe’s outer membrane. This new research shows that at least one methanotroph, Methylosinus trichosporium OB3b, expresses multiple TBDTs to collect not only its MB, but also MB produced by other methanotrophs. Interestingly, expression of the TBDT required for uptake of “foreign” MB increases when M. trichosporium OB3b is exposed to it, indicating that this methanotroph actively seeks to collect MB from other methanotrophs to meet its copper requirements. Moreover, these MB uptake systems collectively regulate expression of the alternative MMOs of M. trichosporium OB3b. Thus, this study indicates that MB uptake, including the uptake of foreign MB, plays an important role in the methane metabolism of M. trichosporium OB3b. These findings provide a novel means by which methanotrophs can be manipulated for a variety of environmental and industrial applications.
Contact
Principal Investigators
Jeremy D. Semrau
The University of Michigan
jsemrau@umich.edu
Alan A. DiSpirito
Iowa State University
aland@iastate.edu
Program Manager
Boris Wawrik
Department of Energy Office of Science
Office of Biological and Environmental Research
boris.wawrik@science.doe.gov
Funding
This work was supported by the Department of Energy Office of Science, Office of Biological and Environmental Research.
Publications
Peng, P., et al., Two TonB-Dependent Transporters in Methylosinus trichosporium OB3b Are Responsible for Uptake of Different Forms of Methanobactin and Are Involved in the Canonical “Copper Switch.” Applied & Environmental Microbiology, 881(2022). [DOI: 10.1128/AEM.01793-21]
Highlight Categories
Performer: University