Summit Charts a Course to Uncover the Origins of Genetic Diseases
Researchers create the most complete model yet of complex protein machinery.
The Science
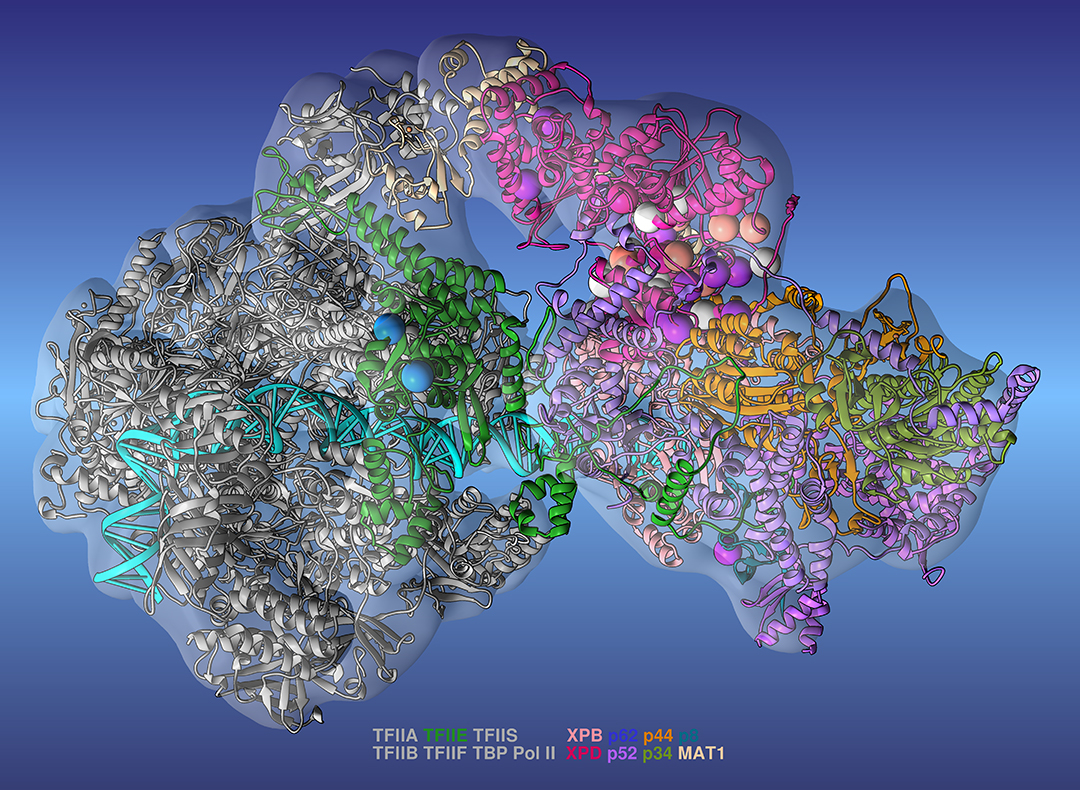
Genetic factors can be responsible for a number of diseases, from degenerative neurological disorders to some cancers. Gene mutations can interfere with how the body expresses genes and cause disease. To better understand this connection, researchers recently developed a model of the transcription preinitiation complex (PIC). The PIC is a group of proteins vital to gene expression. The PIC translates genetic information from DNA to produce proteins and other functional molecules. The simulations revealed how the PIC is organized. It showed that mutations tend to cluster at a specific group of proteins called transcription factor II human (TFIIH).
The Impact
This model could help make sense of the relationship between a patient’s unique genetic makeup and the development of a disease. These findings provided insights into three distinct genetic disorders associated with cancer, aging, and developmental defects. This study provides a foundation for future experimental and computational efforts to delve deeper into the mechanisms of gene expression. Future studies may be able to pinpoint the mutations that cause genetic diseases. Understanding the underlying causes of these diseases could help scientists develop more effective treatments.
Summary
Previous attempts to characterize the PIC have been limited by incomplete models. The team developed the most complete model of the PIC to date. To create this new version, the researchers combined data from cryo-electron microscopy—a structural biology method that uses an electron beam to study cryogenically frozen protein samples—and large-scale molecular dynamics simulations on Summit, the world’s smartest and most powerful supercomputer. This model provides superior insights into the structural organizations of these proteins, which transcribe genes and repair DNA. Because the biochemical pathways responsible for gene expression and repair are intricately intertwined, obtaining detailed information regarding the molecular mechanism behind transcription, the first step of gene expression, is crucial to advancing biomedical applications. The researchers have mainly studied Pol II, an enzyme that transcribes protein-coding genes into messenger RNA molecules that mediate protein synthesis. However, they plan to expand their project to investigate the functional dynamics of Pol I and Pol III, which are known along with Pol II as RNA polymerases, to pursue more groundbreaking insightsContact
Ivaylo Ivanov
Georgia State University
iivanov@gsu.edu
Funding
This work was supported by the National Institutes of Health. The research used computational resources from the Oak Ridge Leadership Computing Facility at Oak Ridge National Laboratory, which is supported by the U.S. Department of Energy’s Office of Science.
Publications
Chunli Yan, Thomas Dodd, Yuan He, John A. Tainer, Susan E. Tsutakawa, and Ivaylo Ivanov, “Transcription Preinitiation Complex Structure and Dynamics Provide Insight into Genetic Diseases.” Nature Structural & Molecular Biology (2019) 26, 397 (2019). [DOI: 10.1038/s41594-019-0220-3]
Related Links
Oak Ridge Leadership Computing Facility News: Summit Charts a Course to Uncover the Origins of Genetic Diseases
Highlight Categories
Program: ASCR
Performer: University , DOE Laboratory , SC User Facilities , ASCR User Facilities , OLCF
Additional: Collaborations , Non-DOE Interagency Collaboration