Supercomputers Drive Discovery of Materials for More Efficient Carbon Capture
Researchers use NERSC to Create Carbon Dioxide-Separating Polymer.
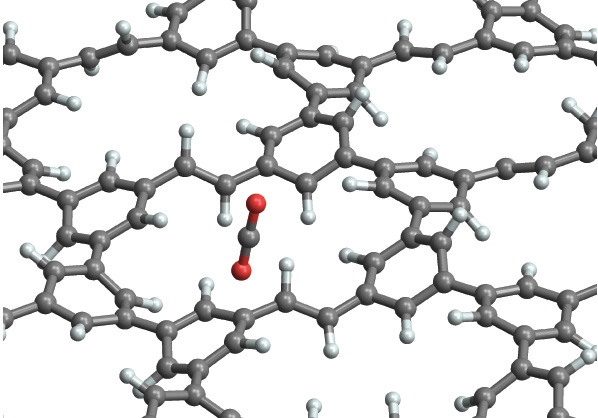
The Science
Using supercomputers at the Department of Energy’s National Energy Research Scientific Computing Center (NERSC), researchers from Haverford College have come up with a new type of two-dimensional polymer which allows, in theory, for highly efficient separation of carbon dioxide. Based on simulations, the material is predicted to be more than 100-times as permeable to carbon dioxide than the best existing materials.
The Impact
Carbon dioxide is the primary greenhouse gas emitted through human activities, such as the combustion of fossil fuels for energy and transportation, and it is widely accepted that it is one of the primary greenhouse gases contributing to global climate change. All of the current approaches to separating carbon dioxide from the exhausts of power plants, which is one way to reduce those emissions, require too much energy to be economically feasible.
Summary
Using supercomputers at the Department of Energy’s National Energy Research Scientific Computing Center (NERSC), researchers from Haverford College have come up with a new type of two-dimensional polymer, PG-ES1, which allows, in theory, for highly efficient separation of carbon dioxide. Based on simulations, PG-ES1 is predicted to be more than 100-times as permeable to carbon dioxide than the best existing materials, while maintaining a rejection of nitrogen and methane gases that meets or exceeds the best existing materials. This allows it to act as a molecular filter that lets the carbon dioxide to pass through easily, while preventing other gases from escaping. Haverford Assistant Professor of Chemistry Joshua Schrier authored a paper on this new material in the most recent issue of ACS Applied Materials and Interfaces. He says the key to the new process is to utilize both the preferential adsorption of carbon dioxide gas molecules on the surface and the ability to create small, nanometer-sized pores in the surface. “Nitrogen and carbon dioxide are linear molecules, and the holes are too small to allow them to enter in any way other than along their ‘skinniest’ dimensions,” says Schrier. “As it turns out, carbon dioxide is a little skinnier than nitrogen, which allows it to pass through the hole more readily. Although it is unlikely that a random molecule would have the correct orientation, the surface adsorption helps increase the local concentration of carbon dioxide and allows each carbon dioxide molecule to try several attempts at different orientations until it finds the correct one, which ‘stacks the deck’ in favor of carbon dioxide passage. Nobody has previously considered the role of surface adsorption on the barrier crossing process, but it is absolutely crucial for performing this type of separation.”
Contact
Joshua Schrier
Department of Chemistry, Haverford College
Haverford, Pennsylvania 19041
jschrier@haverford.edu
Funding
DOE Office of Science Advanced Scientific Computing Research (ASCR) program and a Research Corporation for Science Advancement’s Cottrell Scholar grant
Publications
J. Schrier. “Carbon Dioxide Separation with a Two-Dimensional Polymer Membrane” ACS Appl. Mater. Interfaces, 4 3745–3752 (2012). [DOI: 10.1021/am300867d]
Related Links
Highlight Categories
Program: ASCR
Performer: SC User Facilities , ASCR User Facilities , NERSC
Additional: Technology Impact