Celebrating, Molecular Style

In studying the MOF, the team used microscopes and other instruments at EMSL, a DOE scientific user facility at PNNL.
Trim the guest list or remodel the house? It’s a dilemma that hosts with a small space and a big celebration sometimes face.
Most would try to cut a few names from the guest list. But, a rare molecule has an unusual answer according to a team led by scientists at the Office of Science’s Pacific Northwest National Laboratory (PNNL). The team discovered that this molecule takes the uncommon approach of rearranging its structure to create the room for a diverse guest list. This captivating molecule – known as a soft anionic metal organic framework or MOF – expands its cage-like scaffolding, growing by a third, to take in the molecules it wants.
The molecule is very choosy in the guests it accepts. It has a slightly negative or anionic character – meaning it has a surplus of electrons – so it prefers metals with a positive charge, known as cations. Specifically, it makes welcome guests of metal cations with a double positive charge (technically divalent cations), while ignoring neutral or negative metals.
“To the best of our knowledge, this is the first documented report of capturing metal ions with this class of molecule in the solid state,” said Dr. Praveen Thallapally, a materials scientist who worked on the study at PNNL. The discovery of this molecule’s selectivity, expansion capabilities and easygoing working style provide basic answers about its nature and gives a glimpse of its promise.
“This knowledge is an important step,” said Dr. Jun Liu, who worked on the study and leads transformational materials research at PNNL. “This research could plug into a lot of different areas, including batteries and sensors.”
Discovering the unusually expansive nature of the MOF began with a team from PNNL, University of Edinburgh, and Heriot-Watt University. They synthesized the parent molecule with a zinc base. They added the parent molecule to a liquid containing cobalt. As the team watched, the solution quickly turned violet, indicating that the MOF had captured the cobalt, creating a framework with both zinc and cobalt, which was as expected. Finally, the team added the zinc-cobalt MOF to a liquid containing copper. The solution turned blue as the soft framework also picked up the copper.
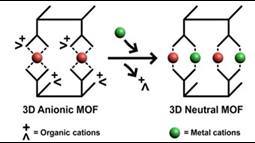
Researchers at Pacific Northwest National Laboratory studied a metal organic freamework with a negative charge and a flexible structure. The framework captured positively charged metal ions, and in the process lost its charge.
The result? A single MOF with three different metal ions: Zinc, cobalt and copper. The positive charges in the metals were counteracted by the negative charge of the framework, and so the material eventually became neutral. “These crystals are unique in that they combine softness and regularity in a single host material,” said Dr. Jian Tian, a postdoctoral research fellow at PNNL and first author on the study.
The team also discovered that they could study the MOF's structure after the guest metals left, and specifically, could measure its internal cavity where the metals resided. Typically, when the guest metal departs, the whole structure collapses. But, with this material, the metals left with minimal fuss, allowing the team to learn how the metals bonded to the host.
As any teen movie will tell you, hosting a gathering with diverse, but ultimately similar, guests can change your life. For the solid MOF, the change may be in the opportunities it receives. Because few solid materials can pack in multiple metals, this MOF could be used in ongoing research into energy storage options, including for wind and solar farms.
This materials research was reported in Journal of the American Chemical Society. For more information, see “Flexible Molecular Cages Expand to Pack in Metals.”
Kristin Manke is a Communications Specialist at Pacific Northwest National Laboratory in Richland, Wash.

