Rich Rewards for Pore Research
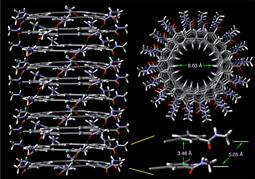 Image courtesy of Bing Gong/University at Buffalo
Image courtesy of Bing Gong/University at Buffalo
A snapshot of a helical stack of macryocycles generated in the computer simulation.
Everyone hopes to get richer…but no one wants to start out poorer. However, many lives could eventually become richer thanks to recent Office of Science supported research on artificial pores.
Pores open possibilities. They serve as controlled gates and filters in cells, allowing water and nutrients and chemical signals in, while keeping out and pushing out waste and other unwanted materials. Pores are useful in other places too, such as chemical filters and water purifiers.
However, it's tough for scientists to build artificial pores that mimic what natural pores can easily do, such as stay together in water and screen materials by size. That changed recently, when an international team of scientists, assisted by the Advanced Photon Source (APS) at Argonne National Laboratory (Argonne Lab), succeeded in creating stable, self-assembling pores which are able to mimic many of the essential features of natural pores.
In a sense, the team found the answer by building better blocks. They started with the element carbon, nature's tinker-toy, a critical backbone of plant walls and animal cells and other essential molecules ranging from fats to fuels. Carbon atoms easily form into stable, six-sided (hexagon) rings, and that's the building block that the researchers used.
Specifically, they used six of those rings connected in a larger ring, a hexagon of hexagons called a ridged macrocycle. Those ridged macrocycles formed the core of the artificial pores, stacking on top of one another in uniform layers.
However, carbon atoms tend to be greasy (and yes, grease also consists of carbon chains), and so the macrocytes needed something more to hold them together. That's why each individual carbon ring in the macrocycle also had ‘mortar' in the form of a side chain containing something called an amide group. Amide groups have slightly positive and negative areas, which attract one another (via hydrogen bonds). Essentially, each slightly positively charged hydrogen atom is attracted to other slightly negatively charged atoms within other side chains.
The attraction kept the pores together, and also ensured that they assembled themselves in uniform structures. Moreover, scientists found that by tacking other atoms or molecules onto individual carbon rings within the larger structures, they could create pores that filter atoms by size, and might even perform specific functions. Such pores can also channel water with few worries. All of that unlocks many possibilities, as proven by the discovery's publication in a recent issue of Nature Communications: http://www.nature.com/ncomms/journal/v3/n7/full/ncomms1949.html
Argonne's APS played an important role in this effort. Scientists used its X-ray beams—the brightest generated by a storage ring in the Western Hemisphere—to confirm the computer simulations that predicted such pores were possible, and then to test their structures layer by layer.
Having proved the concept, scientists and engineers will try to put it into practice. Their creation could eventually grow into a wide range of applications including purifying water, speeding chemical reactions, serving as sophisticated sensors and perhaps even treating some diseases. That's thanks to dedicated research and the Office of Science, whose support of pore research could make many lives much richer.
The Department's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information please visit http://science.energy.gov/about/. For more information about Argonne Lab, please go to http://www.anl.gov/.
Charles Rousseaux is a Senior Writer in the Office of Science.

