A Step Closer to Arresting a Killer
 Wikimedia Commons
Wikimedia Commons
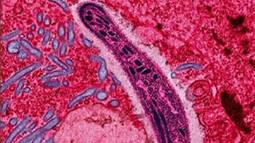
The protozoan Plasmodium falciparum gliding through a cell in the gut of a mosquito, its primary host. Although five different species of Plasmodium can cause malaria, Plasmodium falciparum causes the most severe disease.
It isn't always easy to catch a killer. Doing so often demands a determined effort, a detailed profile, and sometimes, a bright light.
That's what researchers at Washington University in St. Louis are trying to do. The killer they're after is one of the deadliest in history. In 2010 alone, it afflicted an estimated 216 million people, and took some 655,000 lives worldwide, according to the Centers for Disease Control and Prevention.
The killer is malaria. Its deadliest form is caused by an unusual suspect, a microbe with the moniker Plasmodium falciparum. Plasmodium is a parasite, a living cell that depends on other cells to survive and reproduce. It infects people via mosquito bites, and then it breaks into their liver and red blood cells, taking over resources and reproducing, causing misery and often death.
Plasmodium's complicated life makes it tough to catch. However, it does have weaknesses. Like other living things, it depends on protein "machines" to function. One of its important machines goes by the ugly name of phosphoethanolamine methyltransferase, mercifully abbreviated to PMT. PMT is one of the machines Plasmodium uses to build its cellular membrane, the sort of "walls" around its "house." Plasmodium suffers without well-constructed walls: Strains sans PMT live neither long nor well. Importantly, humans don't use PMT, which makes it a natural target for attack.
 Jez
Jez
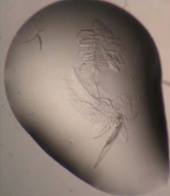
Crystals growing in a drop of liquid protein look more like trapped insects than gemstones, but they’re crystals nonetheless.
However, designing a drug to disable a protein machine requires an understanding of how it works, knowing its structure and finding its active site, the place where a small molecule of medicine might slow it, or shut it down entirely. That's what scientists at Washington University did, with the help of the Office of Science. In a six-year long effort, they determined the structure and the function of PMT.
They first spent years trying to grow crystals of the malarial protein, a frustrating and time consuming process of trying thousands of different combinations, none of which worked quite right. Finally, one did. The researchers then took their precious crystals over to the Structural Biology Center (SBC) beamline (sector 19) at the DOE Office of Science-supported Advanced Photon Source (APS) at Argonne National Laboratory (ANL). The APS provides the brightest X-ray beams in the Western Hemisphere, and can be used to reveal protein structures in incredible detail.
Scientists from around the world use the APS to make discoveries in everything from materials science to medical science. The team from Washington University used the APS to get an X-ray diffraction pattern of the crystal, which gave them the precise structure of the protein, as well as a real sense of how it works.
That information—by itself—won't be enough to stop malaria. But it could lead to new insights which might eventually lead to new medicines. That's doubly important now, at a time when drug-resistant strains of malaria are rising. It's another step closer to arresting a killer, made possible by a relentless search, the Office of Science and an exceptionally bright light.
For more about the APS, please go to: http://www.aps.anl.gov/About/Welcome/. And for more about DOE's Office of Science, please go to: http://science.energy.gov/.
Charles Rousseaux is a Senior Writer in the Office of Science.

